1. INTRODUCTION
In Vietnam, the prevalence of diabetes in Vietnam was 6% in 2013 [1]. The management of diabetes is a multi-disciplined approach. The first-line of drug therapy is metformin and comprehensive lifestyle changes, including weight management and physical activity [2]. The average annual cost of treatment per T2DM patient was 246.1 USD, in which 117.4 USD were spent on treatment of oral antidiabetics [3]. However, some Vietnamese people still prefer to use herbal medicine for their chronic diseases including diabetes mellitus. Traditional Vietnamese medicine is influenced by traditional Chinese medicine. The treatment usually consists of consulting a traditional herbal practitioner following their friends advice (who have used these practitioners), and then adhering to a traditional medicine treatment. In a study by Peltzer, in Vietnam, information on 1601 Vietnamese were collected in urban and rural areas of the Northern provinces on traditional medicine use; 20% of these participants visited herbalists, and 6.2% of respondents had been treated in the previous 12 months for diabetes [4].
The class of biguanides consisting of phenformin and metformin, were first introduced in 1957 as glucose lowering treatment for diabetes management. Phenformin was initially prescribed, but was withdrawn later in many countries in the 1970s because it was associated with lactic acidosis [5]. Lactic acidosis (LA) is a rare adverse effect of biguanide treatment; phenformin use reported a rate of LA of 40 – 64 cases per 100,000 patient-years [6, 7]. Phenformin is still legally available in some countries, such as Mexico, Italy, Brazil, Uruguay, China, Poland, Greece and Portugal, and cases of phenformin-induced lactic acidosis continued to be reported [8]. This reaction caused death in 50% of cases [7]. Metformin was described as an anti-hyperglycemic rather than a hypoglycemic, and the incidence of LA in metformin users was not accurately known, but was estimated to occur in 2 to 9 cases per 100,000 persons-year [6, 9, 10]. Nevertheless, CK Ching [11] reported two fatal cases in Hong Kong of phenformin-induced LA which was found as an adulterant in Chinese proprietary medicine. In 2000, the U.S. FDA recalled Chinese herbal products after discovering high concentrations of phenformin and glyburide [12].
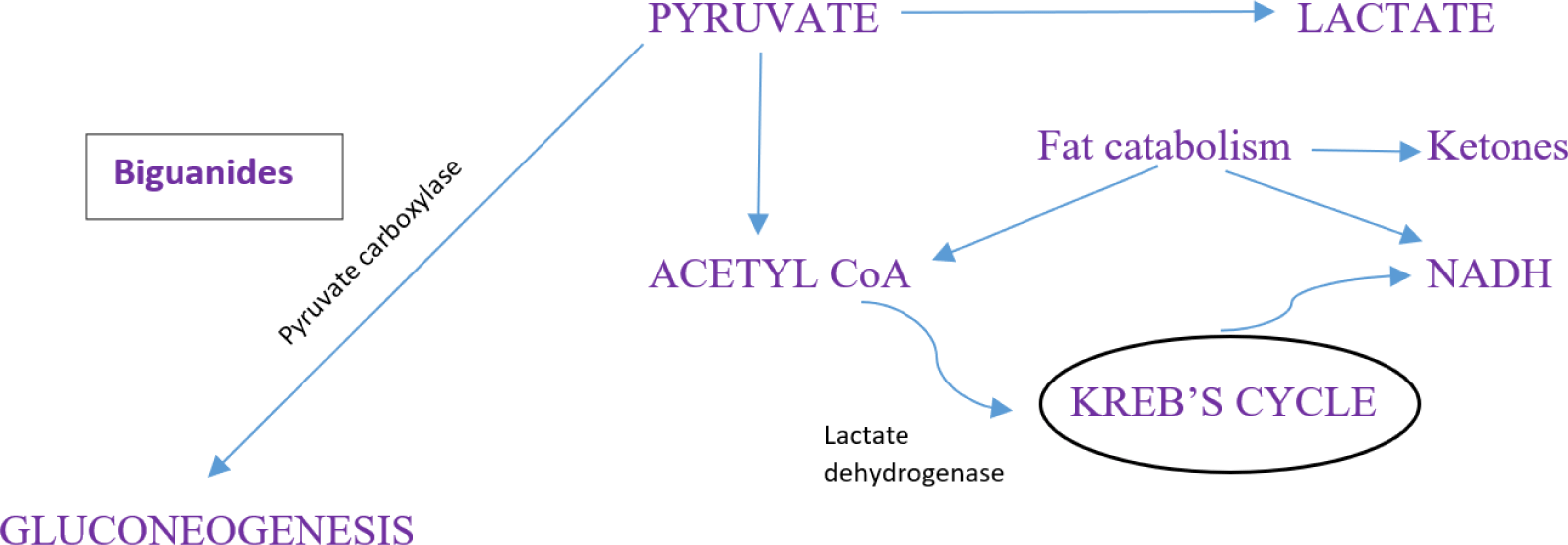
Biguanides have side effects such as anorexia, nausea, and vomiting, resulting in a fasting state that causes fat and muscle catabolism in patients with diabetes. Biguanide-associated lactic acidosis is caused by the inhibition of gluconeogenesis when it accumulates in the cells’ mitochondria. Pyruvate is a metabolite of glucose from glycolysis, which then enters the Krebs cycle during oxidative phosphorylation. Inhibition of this process, leads to a significant reduction in ATP synthesis and an increase in protons, reducing the plasma pH from the continuous hydrolysis of ATP. When the metabolism of pyruvate is blocked, the amount of pyruvate will accumulate and will not be metabolized to oxaloacetate in the mitochondria. It will be converted to lactate which will accumulate, especially in the liver, intestines and a few other tissues [13, 14]. Biguanide toxicity at therapeutic doses does not occur only in patients with renal dysfunction, but also those in heart failure, post-surgical patients, the elderly, with chronic heavy alcohol use, and after use of iodinated contrast. Lactic acidosis can occur due to accumulation [15]. In addition, drug interaction of biguanides with topiramate, zonisamide, acetazolamide or dichlorphenamide, ranolazine, vandetanib, dolutegravir, and cimetidine and alcohol may increase the risk for lactic acidosis.
Recently, herbal anti-diabetic pills have become a preferred choice in Vietnam because of their low price and the belief that they are effective for glycemic control. Severe toxicity and fatalities have been seen with use of these products. We reviewed all cases involving herbal anti-diabetic drugs that were hospitalized with severe lactic acidosis, with or without multiple organ dysfunction syndrome at Cho Ray hospital over a two-year period. The aim of this study was to evaluate the clinical presentation, factors affecting severity, mortality, management, and outcomes of patients admitted after using herbal anti-diabetic medications, and also to determine what substances were contained in these products.
2. MATERIALS AND METHOD
This was an observational and retrospective case series. Data was collected by the first author (Uyen Vy Doan) on all patients who presented with a severe metabolic lactic acidosis after consuming traditional herbal anti-diabetic pills and were admitted to the Emergency Department of Cho Ray Hospital (CRH), from January 2018 through December 2019. Patients included in the analysis had a prior diagnosis of diabetes mellitus, and had previously used metformin before using these herbal anti-diabetic products. Metabolic acidosis is defined as a serum pH ≤ 7.34 (normal range: 7:35-7.42) and serum bicarbonate ≤ 22 mmol/L (normal range: 22–26 mmol/L). Lactic acidosis is defined as metabolic acidosis with an increased anion gap and a lactate concentration ≥ 5 mmol/L (or > 45 mg/dL). Patients not meeting the above criteria were excluded from analysis.
Past medical histories, samples of the herbal anti-diabetic pills, and clinical findings were collected in face-to-face interviews with the author in 18 patients who met the inclusion criteria. All samples of herbal anti-diabetic pills and blood and urine samples (if available), before institution of continuous renal replacement therapy (CRRT) or Intermittent Hemodialysis (IHD) were sent to the toxicology laboratory of the Institute of Public Health to for analysis for the biguanides phenformin and metformin. Two samples were also checked for arsenic. All data of the included cases were retrieved from the medical records of CRH and were entered into Epi InfoTM 7.
The endpoint of treatment was normalization of metabolic parameters, especially lactate (<2 mmol/L or <20 mg/dL), bicarbonate (20 – 25 mmol/L) and potassium (4.0 - 5.1 mmol/L). A metabolic panel was requested every 6 hours on the first day of CRRT (for the patients with hemodynamic instability) or IHD (for hemodynamically stable patients), to prevent overcorrection and ensure that goals were met, and every 12 hours after the first day in the ICU.
Continuous variables were calculated with either mean plus standard deviation (SD) or median plus interquartile range (IQR), and incidence of signs and symptoms were determined. Categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test. All data were analyzed using Stata 12.0 and a statistical significance was established at p < 0.05. This study was approved by the Institutional Review Board of Cho Ray Hospital.
3. RESULTS
Eighteen patients met inclusion criteria, eleven were female (61.1%). The median age of the patients were 59.5 years (IQR 40 – 75 years). The mean duration of a diagnosis of type 2 diabetes was 9 years (SD ± 4.8). There were seven patients with other chronic diseases such as hypertension, cancer, arthritis, COPD, asthma, or vascular diseases. None of the patients had chronic renal failure. The mean duration of herbal anti-diabetic use was 15.6 months; the shortest duration was one month, and the longest duration was 8 years (Table 1).
Two reasons were given for the use of these herbal pills. Patients either chose them for their cheaper price, or their belief in the safety of “natural” plants and herbal medicine. All of the former showed that the prices of the herbal anti-diabetic pills were cheaper than the pharmaceuticals. Many of these patients believed that herbal medicines were safer because their source were plants, and were therefore “natural”. They chose these pills on the advice of their friends with diabetes, who used them with apparent successful glycemic control.
All patients reported gastrointestinal symptoms ranging from 3 days to 3 weeks prior to admission, including nausea, vomiting, abdominal pain and anorexia. Patients also had dry skin, dehydration, and hypotension on admission, with systolic hypotension (<=90 mmHg) in 10 cases (55.6%), and diastolic hypotension (<=60mmHg) in 16 cases (89%). There were two cases of hypoglycemia (blood glucose < 70 mg/dL) on admission (11.2%), and five cases became hypoglycemic during management. On admission, there were eight cases (44.4%) of hyperglycemia (blood glucose > 200 mg/dL); the remaining 8 cases (44.4%), were euglycemic. There were eight cases (44.4%) who were comatose on admission; others were drowsy and confused. Significant acidosis was present in all cases, with the mean serum pH of 6.85 ± 0.21; mean HCO3- was 4.25 mmol/L ± 2.5. The mean serum lactate was 203 mg/dL ± 81), potassium 5.1 ± 1.4 mmol/L, with 12 cases of hyperkalemia. Mean serum BUN was 34.7 ± 9.6 mg/dL, creatinine 2.5 ± 1.08 mg/dL, and mean eGFR was 26.4 ± 10.1 ml/min/1.73m2. Elevated transaminases increased significantly in three cases (>1000U/L); other cases had mild elevation of transaminases. CK-MB tests that were obtained in 12 patients were elevated; the median was 73.75 ng/mL (IQR: 58.4 – 112.7). There was one patient with a significant elevation of CK-MB (6854 ng/mL).
During management, all had severe hypotension; the mean time of vasopressor use was 25 hours ± 28. Continuous renal replacement therapy was performed in 13 patients with hemodynamic instability requiring high doses of vasopressors (noradrenaline, adrenaline, terlipressin). Intermittent hemodialysis was used in 5 patients for correction of acidosis and lactate removal. The duration of continuous renal replacement therapy ranged from 2 to 72 hours, depending on the severity of lactic acidosis and recovery status of each patient. The mortality rate was 33.3 % (6/18 cases) in our study; all of these cases were hypoglycemic on presentation or during admission. Univariate analysis indicated that hypoglycemia during treatment was significantly associated with mortality (P = 0.009). The mean time of recovery from acidosis when Intermittent hemodialysis or continuous renal replacement therapy applied was 28.2 (±18.4) hours. The length of hospital stay ranged from 7 – 14 days.
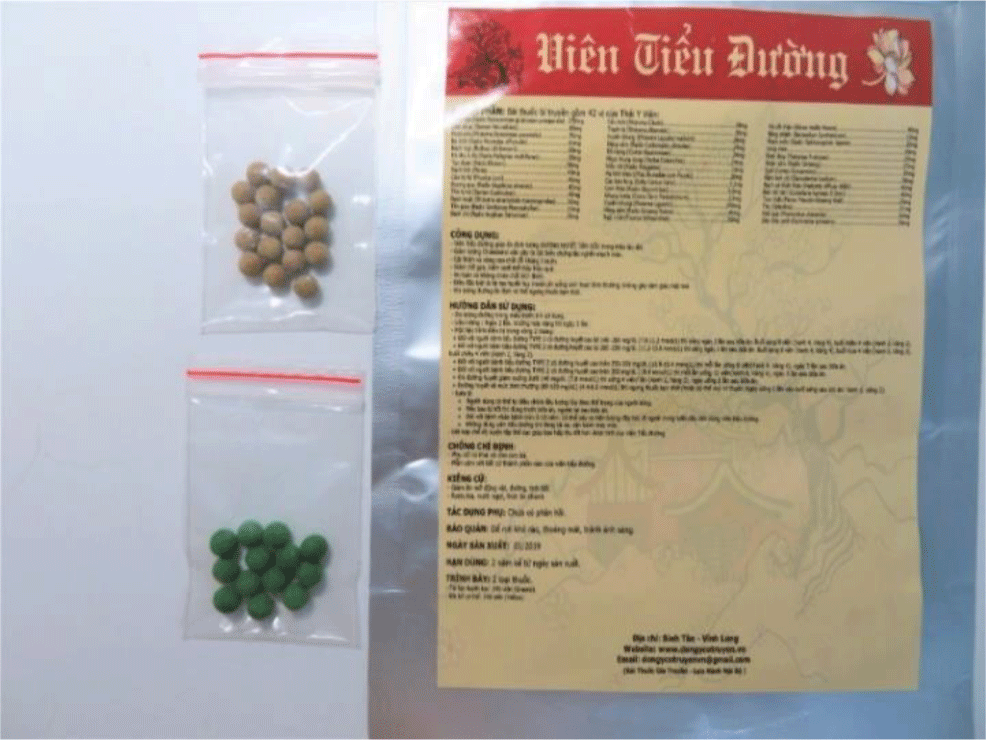
Herbal products used for blood glucose control were collected from 11 patients, with 22 samples which included small green, pink, yellow-brown pills, and black soft balls. There were five samples with metformin and phenformin (with higher phenformin concentrations). Phenformin was found in all samples of herbal pills. The mean phenformin concentration of the pink herbal pills was the highest (15,389.5 mg/kg), compared to the other colored pills. The mean phenformin concentration of the green and brown pills were 7,471.3mg/kg and 32.3 mg/kg, respectively. The highest metformin concentration was detected in a green sample (9,472 mg/kg); the next highest was in pink pills (3,438 mg/kg). The brown pill sample had the lowest concentration of metformin (<10 mg/kg). Arsenic was detected in two samples: the brown pills (0.918 mg/kg), and the black soft balls (0.062 mg/kg). The concentrations of these colorful herbal pills was shown in the Table 2.
Three patients had biguanides detected in whole blood and urine samples before CRRT initiation. Phenformin blood samples were < 0.1mg/L in two patients; urine samples were 6 mg/L and 0.88 mg/L. A third patient had a urine phenformin level of 13.7 mg/L, but was undetected in the blood.
4. DISCUSSION
Herbal medicine treatments for diabetes are popular in Vietnam for some patients who still have believed in traditional herbs because of their efficacy of blood glucose control, and the ease of buying these inexpensive products in traditional herbal stores or temples. These herbal pills can be made by local herbal practitioners or are imported from China.
Our patients had safely used these herbal pills/balls for many months until their date of presentation. Patients followed the herbal practitioners’ guidance, with the belief that these were effective for blood glucose control. Pink pills were used when patients had blood glucose levels > 200 mg/dL. The yellow-brown pills were used for patients with stable glycemia in the range 100 – 200 mg/dL, and the green pills were used for blood glucose levels in the range of 150 – 200 mg/dL. Most patients were advised to combine two different colored pills together, such as green and yellow-brown (for example 4 yellow-brown pills and 2 green pills per dose, twice a day) (Figure 2 and 3). This color combination was appropriate related to the biguanide concentrations of the different colored herbal samples tested. The highest phenformin concentrations were in the pink pills, medium concentrations in the green, and the lowest concentration in yellow-brown pills. In addition, arsenic was found in two samples. Although present in low concentrations, long-term use may result in arsenic toxicity.

In this study, patients have taken these herbal products for blood glucose control for months to years, with the belief that they are effective. On presentation to the hospital, our patients were critically ill. They were commonly misdiagnosed as septic shock with multi-organ failure. With comprehensive past medical histories, we were able to diagnose them as biguanide toxicity, and exclude septic shock. The question in these cases are what were the factors that precipitated this toxicity? These patients had taken these herbal medications for long time before becoming severely ill, with some dying. First, biguanides are still the first choice for diabetes management, However, biguanides are uniquely associated with the development of lactic acidosis, with a mortality incidence exceeding 50% [8, 16, 17, 18]. Second, the most common cause for biguanide toxicity at therapeutic doses is renal dysfunction. However, of note, patients can develop lactic acidosis even if they do not have renal dysfunction [18]. None of the patients in this study had any prior history of renal dysfunction, but they had clinical findings of severe lactic acidosis and multi-organ failure on admission. Third, when taking their medical history, all of them reported having stress in work or in their life at that period of time, bloating, nausea, vomiting, anorexia, and abdominal pain several days to two weeks prior to admission. These symptoms commonly lead to the misdiagnosis of gastritis when patients are seen by their physicians who did not recognized those symptoms were also the side effects of biguanides. Because of this misdiagnosis, patients continued taking these medications. This, in combination with anorexia could lead to hypoglycemia and lactic acidosis, leading to biguanide toxicity. We think that these symptoms appeared while these patients were treating their diabetes and were the “warning signs” for predicting the development of lactic acidosis within days.
Metformin is a routinely prescribed oral anti-hyperglycemic, with a wide safety margin. It is often the first step in treating type II diabetes mellitus. Metformin associated lactic acidosis (MALA) or metformin induced lactic acidosis (MILA) was reported at therapeutic doses [6, 19]. Phenformin is still used in some countries although the incidence of lactic acidosis caused by phenformin is higher than metformin. If traditional herbal medicines only contained ingredient from natural herbs, they would not have any effects on blood glucose control. There is no evidence demonstrating effective blood glucose control from any herbal product. Because of this, traditional medicine practitioners mix biguanides into the herbal antidiabetic products sold in temples or traditional herbal stores in Vietnam. Some patients also believe that the products they bought were imported from China; we identified these from previous studies [11, 20]. When patients used these herbal anti-diabetic products, they were told to combine the yellow-brown pills with green or pink pills. That means the dosage of biguanides were not well controlled and would contribute to biguanide accumulation and toxicity under the right circumstances.
The occurrence of lactic acidosis associated with biguanides has a high mortality rate. The goals of management include measures to enhance perfusion and correction of acidosis. In the ED, our patients were given bicarbonate therapy used for severe acidosis to prevent complications such as cardiac arrhythmias or reduction of cardiac contractility [21]. Close monitoring during sodium bicarbonate administration must be done to avoid complications such as intracellular acidosis, hypervolemia, and electrolyte disorders [9, 22]. Insulin administration can partially reverse the mechanism disorder [23, 24]. This requires close monitoring of blood glucose and serum potassium to prevent hypoglycemia or hypokalemia that can rapidly result in death. Although hypoglycemia is not expected in diabetic patients with biguanide exposure, 11.2 % of the cases in our study had hypoglycemia on admission. It may have occurred because most of the cases in our study had anorexia, nausea, vomiting, insufficient caloric intake alone, or in combination with strenuous exercise or stress at work in the days prior to admission. The rate of hypoglycemia in our study is higher than previously reported [25, 26, 27]. Hypoglycemia was closely associated with mortality in biguanide toxicity in our study (P=0.009 < 0.05), and this finding is different from a study that concluded that hyperglycemia was associated with major outcome or death [25]. The mortality rate in our study was 33.3 % and all of these cases were hypoglycemic on admission or during treatment, even after we had achieved blood glucose control. We did not see any relationship between the duration of use, the number of pills taken daily, underlying chronic disease, and mortality. This could be explained by the interindividual response to biguanides. Biguanides are still effectively used for the treatment of diabetes; metformin is widely prescribed, and phenformin is still approved for use some countries. It was our observation that the influence of external factors such as life stressors, fatigue, anorexia, less appetite in those patients were more likely to develop lactic acidosis.
Hemodialysis was considered for its potential value in enhancing removal of phenformin metabolites, but there is not enough evidence that hemodialysis is effective in removing phenformin [24]. Intermittent hemodialysis or CRRT will improve acidosis and reduce plasma lactate concentration. In our study, there were 13 cases where CRRT was performed (due to hemodynamic instability), and IHD for the 5 patients considered hemodynamically stable enough to tolerate the procedure. Their acidosis and renal function improved in 24 - 48 hours.
Conclusion
Herbal pills are available from herbalists and temples and are used by many for their blood glucose control because they are effective and inexpensive. The herbal pills used by our patients were analyzed for biguanide adulteration. The results showed the presence of phenformin and metformin. Phenformin was present in all samples; when both drugs were present, phenformin was at a greater concentration than metformin. The toxicity seen from herbal anti-diabetic product use was from biguanide adulteration. This occurred in patients with no previous history of renal impairment. Stress and gastrointestinal symptoms were early warning signs of toxicity, which would develop if patients continued with their use. Hypoglycemia was closely associated with mortality in our series of patients presenting with biguanide toxicity.







