1. INTRODUCTION
Budd-Chiari syndrome (BCS), a very rare vascular disease, is defined as the hepatic venous outflow obstruction at any level from the small hepatic veins (HVs) up to the entrance of the inferior vena cava (IVC) into the right atrium [1], The annual incidence of BCS is estimated at 0.13 per million persons in Japan [2], A large case series of BCS in Japan reported that 8% of patients had segmental obstruction of the IVC and HVs [3], The main symptoms include abdominal pain, ascites, hepatomegaly, leg edema and progressive liver failure [4], The disease is frequently accompanied by hypoproteinemia [5], Therefore, BCS is easily misdiagnosed with decompensated liver diseases. Early diagnosis of BCS is very essential due to its high risk of mortality. The 5-year survival rates of 10% and 80% were found in patients submitted to non-treatment and treatment, respectively [6, 7], Along with the advances in radiological intervention, endovascular stenting is currently accepted as an effective treatment [4], We herein report the first case of BCS with segmental obstruction of the intrahepatic portion of IVC and HVs successfully managed by endovascular stenting in Vietnam.
2. CASE DESCRIPTION
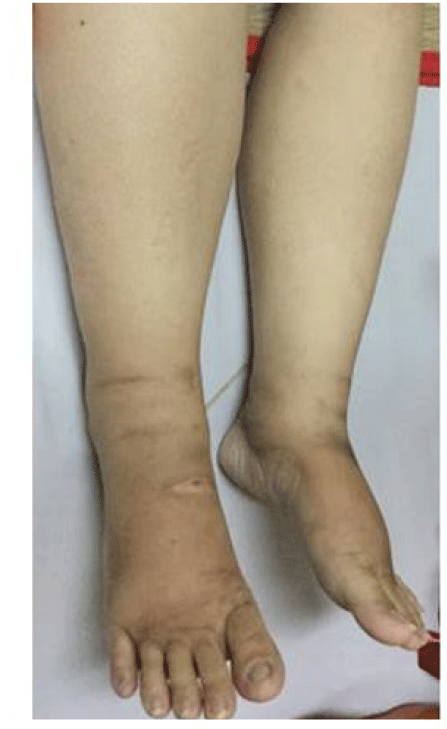
A 32-year-old female patient presented with a 2-month history of progressive ascites and leg swelling (figure 1). She had a history of transcatheter closure of ventricular septal defect at 10 years old. She had no abdominal pain, alcohol consumption or oral contraceptives use. Her family history was unremarkable. Massive ascites without the collateral system of the abdominal wall and marked lower extremity edema were recognized. The slightly enlarged, firm liver and spleen without tenderness were present. There were no signs of liver failure.
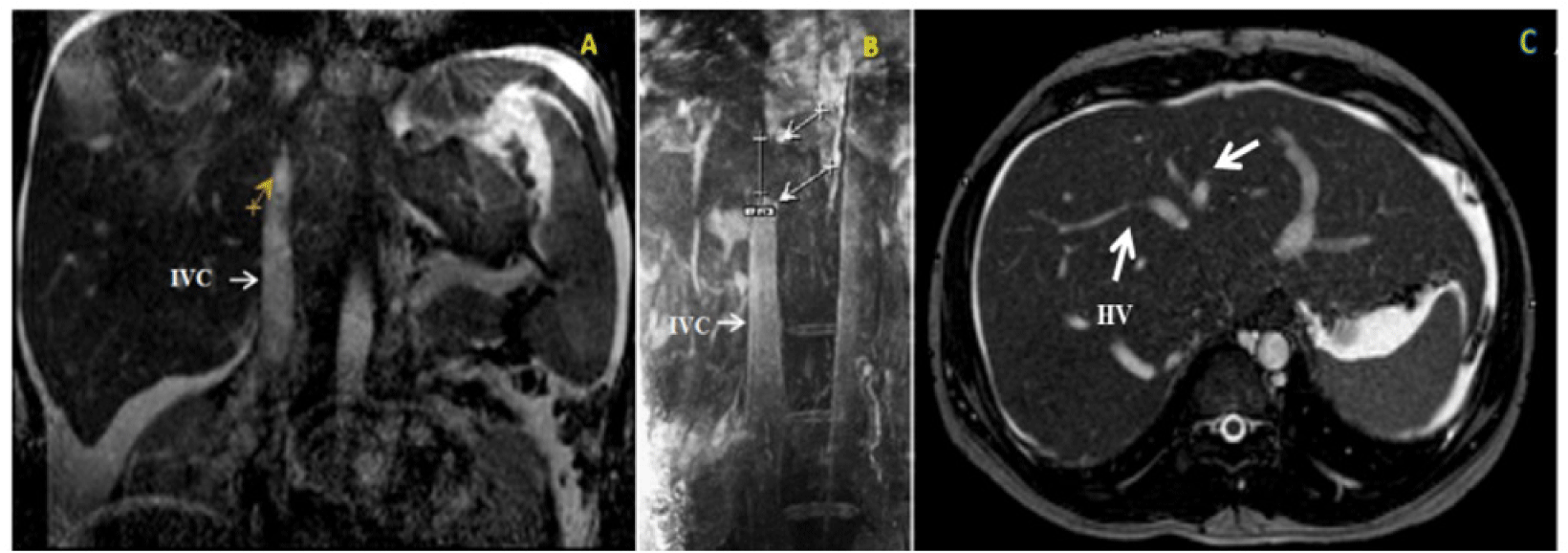
A complete blood cell count showed polycythemia (hemoglobin of 16.1g/dL and hematocrit of 48.7%), normal white blood cell and platelet count. The serum electrolytes and renal function tests were normal. Liver function tests included: serum albumin level of 2.4 g/dL, total serum protein concentration of 4 g/dL, total bilirubin level of 1.4 mg/dL, international normalized ratio (INR) of 1.37 and normal transaminase level. Proteinuria was negative. The venous ultrasound of the lower extremity showed mild varicose veins. The ascitic fluid was transudate with no evidence of infection. Ascitic fluid analysis included: adenosine deaminase of 12.6 IU/L, serum-ascites albumin gradient (SAAG) of 1.9g/dL, protein of 1.1 g/dL and glucose of 7.51 mmol/L. Abdominal computed tomography (CT) scan suggested the image of cirrhosis due to hepatosplenomegaly and massive ascites. Abdominal doppler ultrasound and magnetic resonance imaging (MRI) showed the 3-cm long segmental IVC blockage and the 2-cm long segmental HVs stenosis without thrombosis (figure 2). Therefore, our final diagnosis was BCS with segmental obstruction of the IVC and HVs.
Further tests have been ordered to investigate the cause of BCS. The morphologic examination of erythrocytes in peripheral blood smear and serum erythropoietin level were unnoticeable. Unfortunately, JAK2 mutation testing and red cell mass measurement were not performed because of unavailable local facility. Protein C, Protein S and antithrombin levels were normal. HBsAg, anti HCV, ANA, anti dsDNA were all negative. Tumor markers including AFP, CEA, CA 125, CA 15-3 and CA 19-9 were in normal range. In addition, chest X-ray, upper gastrointestinal endoscopy, colonoscopy, genital ultrasound, echocardiogram and bone marrow biopsy results were unremarkable. However, small bowel endoscopy to look for causes of protein-losing enteropathy was not accomplished due to lack of patient’s acceptance. Therefore, there was no evidence that the BCS case was related to hypercoagulation or malignant disorders.
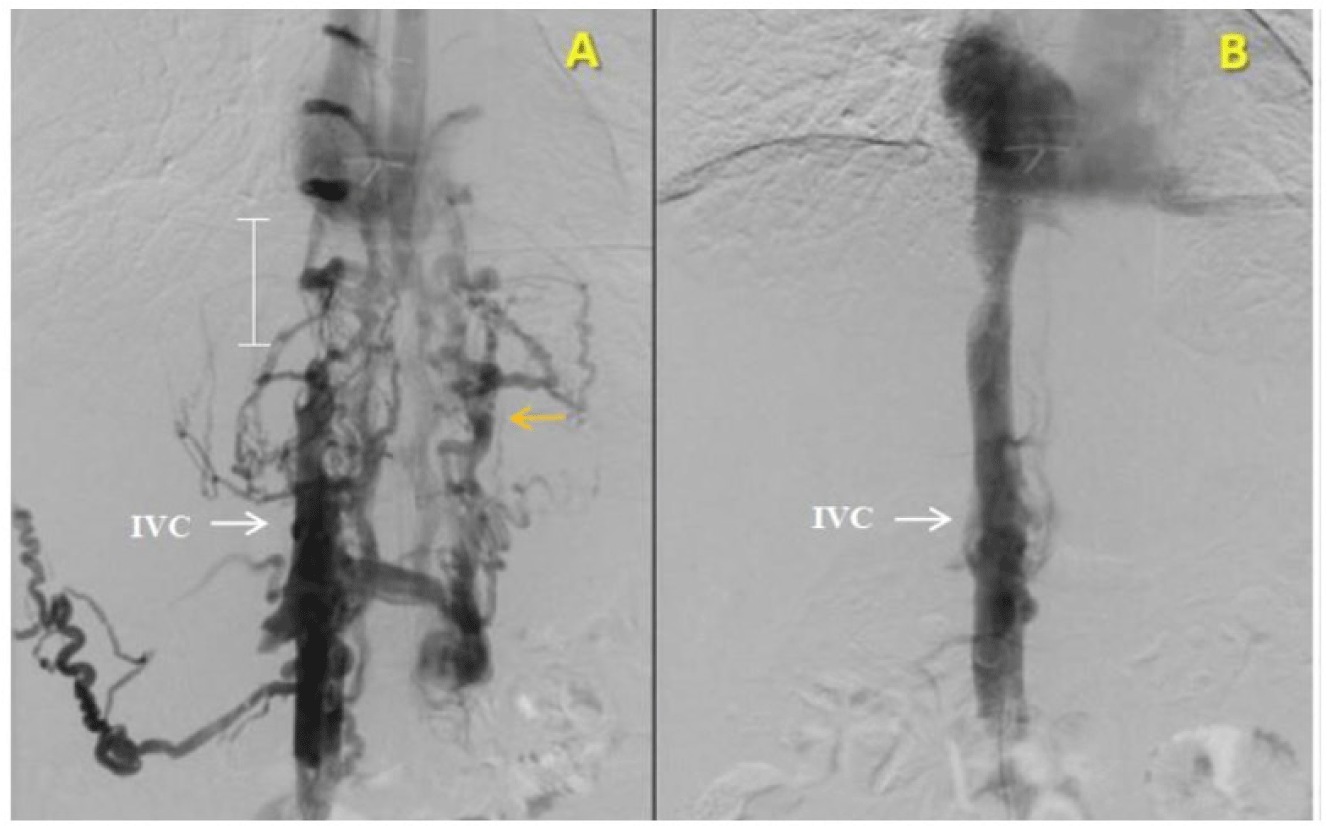
Recanalization by balloon dilation and endovascular stenting were performed. Under general anesthesia, a 6F sheath and a 5F pigtail catheter were advanced into IVC through the right femoral vein. Unfractionated heparin (5000 units) was given. Venography revealed a 3-cm long segmental obstruction of the intrahepatic segment of IVC with drainage of extensive collateral circulation (including azygos vein, renal vein and vertebral vein) into superior vena cava (SVC) (figure 3A). No hepatic vein was visualized. The segmental obstruction of IVC was accessed with a 0.035” guidewire and was dilated with 5 x 40 mm Mustang and 10 X 40 mm Conquest balloons. It was then stented with 14 x 160 mm Protégé stent. Venogram post stenting showed good stent apposition and good flow of IVC into right atrium without collateral circulation (figure 3B). No complication related to the procedures was noted.
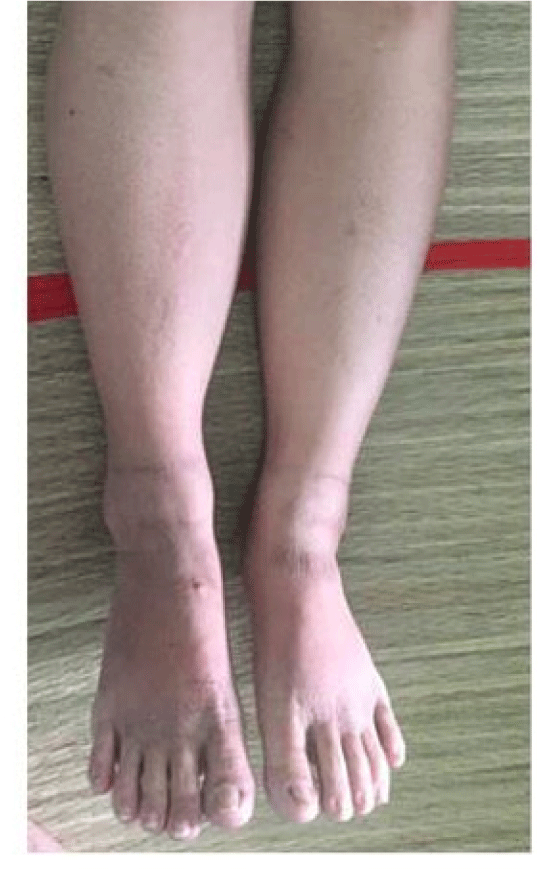
After recanulization, IVC pressure dropped significantly (from 31 mmHg to 21 mmHg), thus portal hypertension also improved. The patient was given lifelong anticoagulants, initially with heparin and followed by warfarin titrated to maintain INR between 2 and 3 to avoid stent thrombosis. Leg swelling and ascites were resolved completely in 3 days (figure 4). She was discharged 3 weeks after admission with remarkable improvement of clinical condition. During 1-year follow-up, ascites and leg edema were not recurred and laboratory results were all normal. Besides, restenosis of IVC and stent malapposition were not also found.
3. DISCUSSION
Diagnosis of BCS is a challenge because of its various presentations and rarity (1/100,000 of the general population worldwide) [1, 7, 8]. BCS is usually suspected if patients have one or more of the following symptoms: (a) classic triad of abdominal pain, ascites, hepatomegaly; (b) abrupt onset of ascites and painful hepatomegaly; (c) massive ascites with relatively preserved liver functions; (d) unexplained chronic liver disease; (e) liver disease with thrombogenic disorder; (f) fulminant hepatic failure associated with hepatomegaly and ascites; or (g) sinusoidal dilation in liver biopsy without heart disease [1, 9, 10]. Besides massive ascites, our 32-year-old patient suffered from lower extremity edema, hepatosplenomegaly and mild liver dysfunction. After exclusion of other causes of ascites, BCS was differentiated from decompensated cirrhosis. Because of no signs of liver failure (jaundice, asterixis, palmar erythema, etc.), polycythemia instead of anemia and the discrepancy between mild liver dysfunction and severe ascites and leg swelling, BCS was more likely a diagnosis for this patient. However, the difficulty in this case was that the patient had atypical manifestations of BCS (ascites together with leg edema, liver failure, spleen enlargement, but no abdominal pain). According to Murad [10], the percentage of abdominal pain was only 61%. Her mild liver dysfunction and hepatosplenomegaly could be caused by longtime portal hypertension. Varicose vein of the leg could cause leg swelling. However, mild varicose veins on venous ultrasound of the legs were not compatible with the patient’s marked edema. Therefore, the etiologies of edema were not only varicose veins of the legs but also BCS. The most striking presentation of HV obstruction is ascites, whereas the most outstanding symptom of IVC compression is leg edema [1, 11]. As a result, both lower extremity edema and ascites were mainly related to IVC and HVs obstruction, which was accordant with the patient’s MRI results. Perhaps the portion of IVC blockage was longer than its HVs obstruction, so that her leg swelling was more impressive than her ascites. In the unclear situation, the predisposing thrombophilic factors are important and helpful for diagnosis of BCS.
Thrombogenic risk factors should be investigated because at least one prothrombotic disorder is present in 80% of patients with BCS and 50% of them have more than one [12] , These include oral contraceptives use, pregnancy within 3 months before diagnosis, myeloproliferative disorders (polycythemia vera, thrombocythemia), deficiencies in protein C, S, malignant or immunologic diseases [12], Among these risk factors, polycythemia vera was suspected to be related to our case. Although JAK2 mutation testing and red cell mass measurement were not performed, bone marrow biopsy result, peripheral blood smear result and serum erythropoietin level were all normal. In addition, red blood cell count slightly increased. According to the World Health Organization classification of myeloid neoplasms [12] , the patient did not meet criteria for polycythemia vera. After screening other causes of polycythemia, we confirmed reduced plasma volume was the etiology of elevated red blood cell count. Most of the above risk factors were listed in European prospective cohort studies, while unknown risk factors were reported in Asian researches [10, 14], Perhaps this difference could be related to the unequal distribution of causes of BCS among continents. Pure HV block due to thrombosis or malignant tumor is more prevalent in Europe, whereas pure IVC or combined IVC/HV block due to web or fibrous cord is more common in Asia [1, 15], The block of IVC is frequently associated with the intrahepatic portion [16-18], Origin of the fibrous cord has been unknown but two hypotheses have been suggested. Firstly, it is a congenital vascular malformation of IVC during embryonic development, especially intrahepatic portion of IVC at diaphragmatic level. Secondly, it is the transformation of the IVC thrombosis into membranous or fibrous occlusion [3, 11, 19], No study on risk factors of BCS has been currently published in Vietnam. This is the limitation of the case report because the thrombotic risk factors of BCS in Europe may be different from the ones in Vietnam. In case of high clinical suspicion, the physian should evaluate symptoms in detail, elicit the other causes of ascites and indicate imaging facilities for definitive diagnosis.
The diagnosis of BCS is established based on radiological imaging of HVs and/or IVC obstruction [11, 15], CT scan may be helpful with the presence of regenerative nodules, delay of filling of the contrast medium in the HVs and/or IVC, as well as unequal parenchymal contrast enhancement between peripheral and central regions of the liver [7, 20], Unfortunately, our patient’s CT scan result was equivocal, so that BCS was mistaken with cirrhosis. According to Gupta [21], false positive and indeterminate results can occur in 50% of cases. Due to the inconsistency between clinical data and imaging result, some special imaging studies were indicated. Doppler sonography has an accuracy of nearly 85% and should be the initial investigation when BCS is suspected [1]. In case of unremarkable ultrasound, MRI is a reasonable option. Hepatic venography, the gold standard for BCS diagnosis, is also helpful to evaluate the extent of HV/IVC thrombosis and caval pressures. It, therefore, should be performed when BCS suspicion is high and other imaging techniques have failed [1]. Regard to anatomy, HV is the only outflow vessel from the liver to the IVC and right atrium. Small accessory HVs such as vertebral vein, renal vein and azygos - hemiazygos vein, which direct towards 20% of IVC, contribute to collateral venous drainage from common iliac vein to SVC in case of HVs or IVC obstruction [8, 19], It is consistent with the patient’s hepatic venography results (Figure 3A).
Unlike imaging studies, liver function tests and ascitic fluid analysis contribute little to make the diagnosis of BCS, but they can be helpful to assess the stages of BCS [1, 7], Most of patients usually have a high SAAG (> 1.1 g/ dL) in combination with a total ascitic protein level above 2.5 g/dL. It is possible not to present in patients with acute BCS (ascitic protein < 2g/dL) [1, 14], Interestingly, unlike literature reviews, the patient’s serum and ascitic protein level were very low although BCS appeared in subacute form. The proportion of BCS with hypoproteinemia was reported in about 27.1% in Japan, and 38.5% in western countries [5], The explanations include microcirculatory disturbances, increase in vascular permeability and disorder of lymph circulation which cause loss protein in ascitic fluid or gastrointestinal tract [5], In addition, hypoproteinemia may be due to liver failure. Ascitic protein level depends not only on the severity of portal hypertension but also on serum protein concentration [22], Perhaps mild portal hypertension due to extensive collateral circulation and hypoproteinemia could cause low ascitic protein level.
In Vietnam, we have currently found a few cases of BCS reported at the national conference. All cases caused by thrombosis of HVs were mainly treated by anticoagulants. This is the first case of BCS with segmental obstruction of the IVC and HVs successfully managed with endovascular stenting. Especially, she also had low serum and ascitic protein level and liver failure. It could be easily misdiagnosed with decompensated cirrhosis. The critical key of the early diagnosis of BCS is that physicians need consider the relationship between symptoms and laboratory results systemically, not only base on routine imaging or ascitic fluid analysis. In case of high suspicion of BCS, it is essential to indicate special imaging studies such as doppler sonography, MRI and hepatic venography.
The goals of treatment were to recanalize venous obstruction, prevent extension of thrombosis in the IVC/HVs and preserve hepatic function by decreasing the centrilobular congestion. Currently, there is no consensus on the management strategy of BCS because it depends on etiology, location and extent of venous obstruction as well as available local facilities and experts [1]. Methods of treatment include angioplasty, transjugular intrahepatic portosystemic shunt or surgery. Recently, endovascular stenting has been reported as an effective treatment [23, 24].The initial successful rate of stenting was 90% without major complications, and the longterm patency rate was 75% [24], Our patient was managed with balloon dilation and endovascular stenting. The clinical and laboratory improvement were significant within 3 days after stenting and maintain normal during 1-year follow-up.
4. CONCLUSION
BCS with segmental obstruction of the IVC and HVs is a very rare condition. The challenge for early diagnosis is that its symptoms are quite similar to features of liver diseases. The diagnosis should be based on a high clinical suspicion in combination with diagnostic imaging facilities. Despite being an invasive technique, hepatic venography provides useful information when all non-invasive imaging techniques only provide equivocal results. Endovascular stenting is a promising option for recanalization of IVC occlusion in case of the failure of medical treatment and unavailable liver transplantation.
LEARNING POINTS:
-
BCS, a very rare and life-threatening disease, is curable if early diagnosis and appropriate treatment are performed.
-
BCS with hypoproteinemia and low ascitic protein level are easily misdiagnosed with chronic liver diseases.
-
Early diagnosis should be based on a high clinical suspicion in combination with special imaging studies (doppler sonography, MRI and hepatic venography).
-
Endovascular stenting is currently accepted as an effective treatment in case of unavailable liver transplantation.