1. INTRODUCTION
Septic shock is the most common type of shock in the intensive care unit (ICU), accompanied with high mortality. Hemodynamic disturbances in septic shock are characterized by venodilation, resulting in venous blood pooling and capillary leakage, leading to reduced intravascular volume, at least in the early phase [1, 2].
Fluid resuscitation, therefore, plays a crucial role in restoring intravascular volume and ensuring tissue perfusion, especially in the rescue phase. In fact, previous protocols based on aggressive fluid administration during the early phase of septic shock showed significant improvement in patient outcome [3, 4].
However, fluid administration in the later phase of shock demonstrates less clear benefit and even possible harm. In the previously published VASST study [5], patients with the highest positive fluid balance were associated with the highest mortality. Moreover, the negative effect of positive fluid balance occurred very early at 12 hours. More recently, Kelm et al. did a study in which patients were treated using early goal-directed therapy. This demonstrated that fluid overload presented in almost 70% of cases [6], who needed more intensive medical interventions and continuous renal replacement therapy, and had higher mortality.
After initial rescue phase, fluid management aims to keep balance between hypo-perfusion due to under-resuscitation and circulatory overload [7]. In this optimization phase, fluid is often prescribed when hypovolemia is clinically suspected, e.g. hypotension, low filling pressure, low urine output (UOP), or prolonged capillary refill time.
Bihari et al., found 95% of patients who were completed the initial 6-hour bundle of resuscitation in a study of Bihari et al. still received fluid bolus (FB) on the first day and 52% on the second day [8]. Low filling pressure was the most effective trigger, and increasing vasopressor requirement was the least effective one. However, in this study, the effectiveness of FB was evaluated only by clinician judgement without measuring hemodynamic parameters.
On the other hand, in patients with acute respiratory distress syndrome (ARDS) due to sepsis, there was no difference in the fluid responsiveness between bolus triggers of low blood pressure, low filling pressure and oliguria when evaluated by cardiac index [9].
In studies of Bihari and Lammi [8, 9], FB could be repeated but effectiveness among them was not evaluated separately. Furthermore, the combination of these triggers seemed to be used very often but the responsiveness of the interventions was not explored in detail [10].
Our purpose was to evaluate the fluid responsiveness according to different FB triggers and their combination in the optimization phase of septic shock management.
2. METHOD
Patients from two teaching hospitals in Ho Chi Minh City, Vietnam (HCMC) were consented and enrolled prospectively from October 2014 to February 2016. The database of our study was adapted from the trial evaluating fluid effectiveness in severe sepsis and septic shock, which was approved by Ethics Committee of the University of Medicine and Pharmacy in HCMC. This study used stroke volume (SV) to assess fluid responsiveness in the late phase of shock resuscitation and some parts of it was published on a local journal. Verbal informed consent was obtained in some patients and written informed consent was obtained from all patients next of kin for insertion of central venous catheter and intra-arterial catheter, and FB.
We included adult patients (2: 18 years old) who were diagnosed with severe sepsis and septic shock according to the Society of Critical Care Medicine criteria [11] and had at least one FB triggers: heart rate > 100 bpm, UOP < 0.5 ml/ kg/h, serum lactate level > 2 mmol/l, central venous pressure (CVP) <12 mmHg (if spontaneous breathing) or < 15 mmHg (if mechanical ventilation), and mean arterial pressure (MAP) < 65 mmHg. Exclusion criteria were: arrhythmias, signs of circulatory overload (e.g. respiratory crepitation or CVP > 15 mmHg), change of vasopressors dose or ventilator settings during fluid administration, and contraindications of insertion of central venous catheter and trans-radial arterial catheter.
All patients were placed an internal jugular vein catheter to measure CVP and a trans-radial arterial catheter to measure directly blood pressure. CVP was measured by Truwave transducer (Edwards Lifescience) and monitor Lifescope 3500 (Nihon Kohden). Blood pressure and SV was measured using uncalibrated arterial pressure waveform analysis by FloTrac sensor and monitor EV1000 (Edwards Lifesciences). The FloTrac system was proved to be reliable enough to track SV changes induced by FB [12].
FB was performed by 500 ml Ringer lactate infused over 30 minutes. SV was measured before and right after FB. FB responder was defined as an increase 2: 15% in SV after FB. If SV increased less than 15% and the bolus triggers still existed, FB will be repeated one more time.
Patients age, gender and Acute Physiology and Chronic Health Evaluation II (APACHE II) score were collected and computed from patient medical record. Sources of infection were identified according to the criteria of the International Sepsis Forum [13].
Continuous variables such as age, APACHE II score, MAP were described as mean ± standard deviation (SD) (if normal distribution) or median and interquartile range (IQR) (if non-normal distribution). Discrete variables such as source of infection, severity of sepsis was presented as percentages. Fluid response rate of the first and the second bolus were compared by Chi-square (**2) test. The comparison of hemodynamic parameters before and after fluid infusion was analyzed using Student’s t-test or Mann-Whitney test when appropriated.
Because the bolus triggers may not be independent, multivariable logistic regression adjusted to bias factors (age, gender, APACHE II score, shock state, and mechanical ventilation) was used to explore response rate according to pre-specified cut-offs (UOP < 0.5 ml/kg/h, serum lactate level > 2 mmol/l, CVP < 8 mmHg, MAP < 65 mmHg). On the other hand, response rate according to number of triggers was examined by logistic regression using response rate to one trigger as reference.
P value < 0.05 was considered statistically significant. All analyses were performed using STATA 14.0 (StataCorp. LLC, Texas, USA).
3. RESULTS
Eighty-four patients enrolled in this study had clinical characteristics presented in Table 1. Mean age was 63.5 ± 14.7 years old. More than 60% were male. Approximately 70% of patients had septic shock. Mean APACHE II score was 19.5 ± 7.3. Fifty-seven patients were prescribed vasopressors, and norepinephrine was used mostly (56/57 cases). The two most common sources of infection were abdomen and respiratory tract. Most of FBs were performed on the second day of sepsis (1.63 ± 0.98 days).
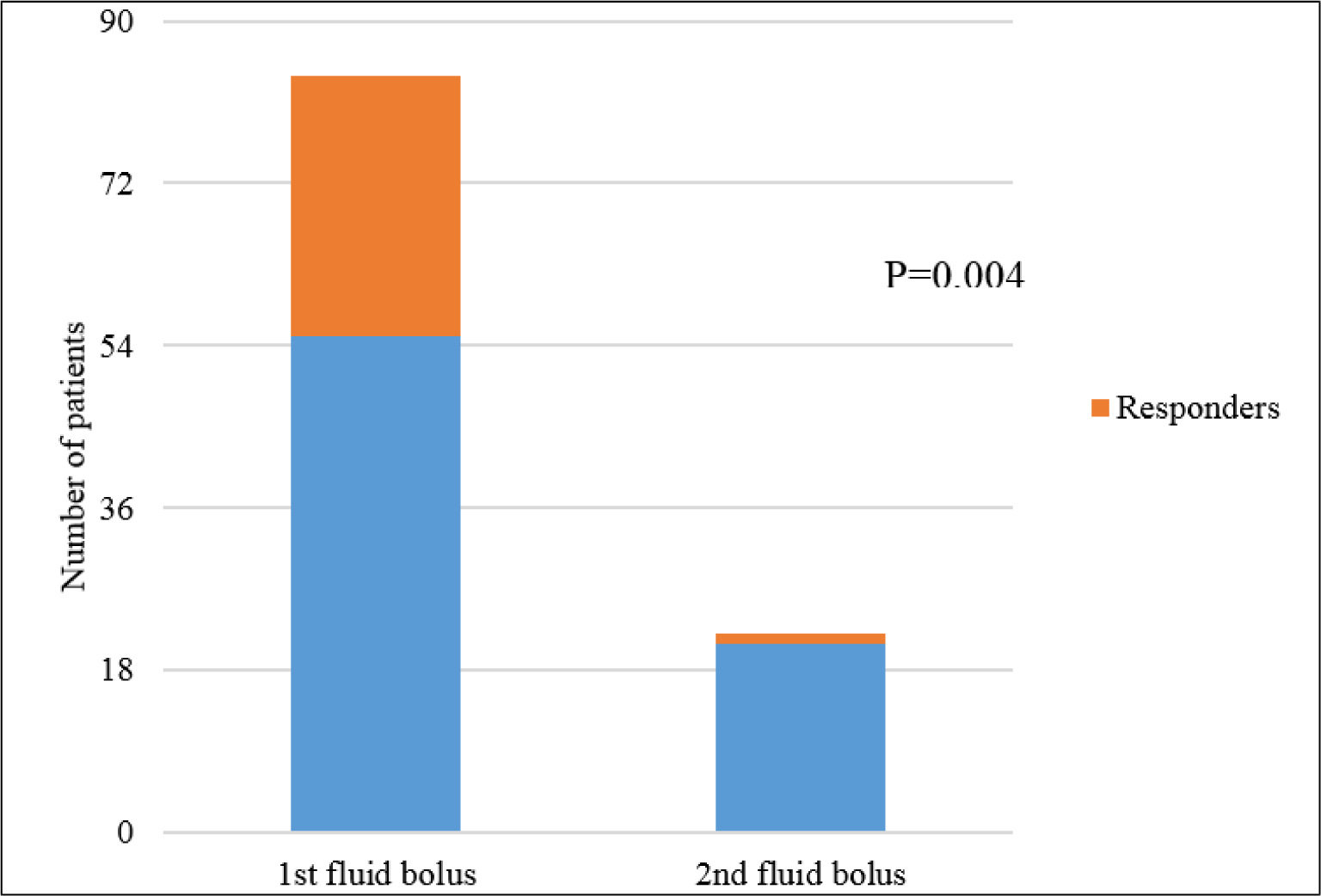
Thirty patients responded to FB, and 29 of them responded to the first one (34.5%). However, in 22 patients who received the second FB because the bolus triggers still existed, only one patient responded (4.5%), making the response rate to the second FB lower than the first bolus (p = 0.004) (Figure 1). The hemodynamic profile before FB was similar between responders and non-responders (Table 2), except CVP was lower in responders (7.3 ± 3.4 mmHg vs. 9.2 ± 3.6 mmHg, p = 0.018).
FBs were mostly prescribed for tachycardia (65 times), followed by high serum lactate level (57 times), whereas low blood pressure was the least used trigger (19 times). CVP and UOP were used for FB indication 35 and 27 times, respectively.
Response rate of the bolus triggers were: low CVP (48.6%), low MAP (47.4%), tachycardia (38.5%), low UOP (37%) and high lactate level (36.8%). Giving fluid for low CVP (i.e. CVP < 8 mmHg) was the most effective trigger with adjusted OR 2.81 (95% CI 1.08 - 7.27) (Table 3). However, the association was no longer significant with lower cut-offs in sensitivity analysis, e.g. CVP < 7 mmHg (adjusted OR 2.42, 95% CI 0.91 - 6.42), CVP < 6 mmHg (adjusted OR 1.62, 95% CI 0.54 - 5.29).
FB was usually given when patients had two or three bolus triggers. Considering patient with 1 trigger as reference group, we noticed there was a trend of higher response rate, however it did not reach statistical significance (Table 4).
4. DISCUSSION
The guidelines published in 2012 by the Surviving Sepsis Campaign recommends early aggressive fluid therapy in the initial phase of shock to correct tissue hypo-perfusion [11]. It also emphasizes more cautious use of fluid in the late phase of shock, that requires continuous monitoring hemodynamic status and patient response. In our study, enrolling patients during the optimization phase of septic shock, we have illustrated that fluid responsiveness was low demonstrated by: (a) only 35.7% of patients responded to FB (i.e. increase SV ≥ 15% after FB), (b) responsiveness to the second FB was lower than to the first one, (c) neither FB triggers nor their combination provided higher responsiveness.
Our study adds to the growing evidence suggesting that FB performed in late phase of shock in patients who have previously received fluid is associated with limited success. In a study, which enrolled patients who were completed 6-hour resuscitation bundle, Bihari et al. demonstrated that the two most common indications for FB were low blood pressure and increasing vasopressors dose with the frequency of response 37.1% and 29.7% respectively [8]. Interestingly, FBs in their study were used with larger volume (e.g. 750 ml for the first FB) and deemed to be successful based on physician perception but the response rate was similar in our study. More recently, using cardiac index changes to evaluate fluid responsiveness in ARDS patients who were resuscitated with IV fluid, Lammi found only 23% of patient responded to FB [9].It is noteworthy that using a high cut-off of SV changes to define fluid responsiveness and low bolus volume may result in the low rate of fluid responsiveness in our study. We believe that any method to perform and evaluate FB in the late phase of septic shock is unlikely to be successful.
After the 6-hour bundle, in which at least 30 ml/kg of crystalloid was administered, more fluid could be given based on hemodynamic status [11]. In fact, patients received on average 3 FBs during their first 4 days in ICU according to a survey in French, or 4.5 ± 3.9 boluses in FACTT study [9, 10]. In 22 patients who did not respond to the first FB and triggers for FB still existed, we performed the second FB. Only one of these patients responded to the second FB resulting the response rate (4.5%) which was significantly lower than the response rate to the first FB (34.5%) (p 0.004). Performing fluid loading in post cardiac surgery patients with 200 ml of fluid every 10 minute up to 1800 ml, Trof noticed the increases of cardiac index at 30-minute, 60-minute and 90-minute were gradually decreased [14]. The frequency of responding events (increase of cardiac index ≥ 10%) also decreased from t = 0 to 90 minutes. In order to explore the mechanism of lack of fluid responsiveness, Gupta measured mean systemic filling pressure and right atrial pressure before and after FBs. The second bolus raised the mean systemic filling pressure but also right atrial pressure in non-responders, resulting in the changes of this gradient pressure (mean systemic filling pressure minus right atrial pressure) significant lower compared to its changes in responders, 0.30 (-0.45 to 1.1) and 1.1 (0.12 to 2.0) mmHg respectively [15]. This not-increasing of gradient pressure for venous return coupled with myocardial dysfunction which is common in septic shock may explain for a very low response in patients who already failed the first bolus.
In optimization phase, fluid is usually administered upon clinical signs that suggest of hypovolemia, i.e. fluid triggers. Among these triggers, tachycardia was the most common used in our study. In a meta-analysis about diagnostic value of vital signs in hypovolemic state, heart rate > 100 bpm had a very high specificity in revealing hypovolemia due to blood loss [16]. Although being considered as a form of hypovolemic shock, unlike hemorrhagic shock, tachycardia in septic shock may be caused by many other etiologies such as hyper-metabolism, effect of cytokines on sinus node and vasopressor use turns it to be a unreliable indicator of hypovolemia [17]. In our study, patients with tachycardia had a higher response rate than patients without tachycardia (38.5% versus 26.3%), but this difference was not statistically significance.
CVP is an easily measured and commonly used to predict fluid responsiveness. Using a low cut-off (≤ 8 mmHg) in Surviving Sepsis Campaign 2012 guidelines we found the response rate to low CVP (48.6%) was highest compared to other triggers with OR 2.81. However, in sensitivity analysis, we did not find higher response rate at lower cut-offs of CVP which indicated the former cut-off was likely to have been a spurious finding. Our result is consistent with a meta-analysis that demonstrated CVP neither relate to intravascular volume nor predict fluid responsiveness [18].
Besides low filling pressure, low blood pressure also had high response rate (47.4%). In early phase of septic shock, hypotension is a sign hypovolemia but it is mainly due to low vascular tone [1, 19]. Therefore, fluid responsiveness according this trigger was not higher than other triggers.
Oliguria is another traditional sign of hypovolemia that is often used to trigger fluid administration. Bihari reported it was the bolus with lowest responsiveness among FB triggers while it was more effective than low filling pressure in ARDS patients [8, 9]. We found oliguria was the least used trigger and its responsiveness was lower than with low CVP and low MAP. It suggested that clinician was aware of the limitation of this parameter in predicting volume status. In fact, UOP in septic shock is controlled by renal mechanism more than hemodynamic parameter makes oliguria become a unreliable indicator of hypovolemia [20].
High lactate level is also considered as manifestation of hypovolemia/hypo-perfusion and target of therapeutic strategies aiming to improve oxygen delivery [11]. We used lactate level ≥ 2 mmol/L to trigger FB but found that it was the least effective one. A study of Ospina-Tascon suggested that the relationship between lactate and macro hemodynamic index, if there was, just existed in early phase of septic shock [21]. However, more evidence showed that sepsis-associated hyperlactatemia not only caused by hypo-perfusion and anaerobic metabolism but also by accelerated adrenergic-driven aerobic glycolysis [22].
Besides individual FB triggers, we also evaluated the combination of these triggers in fluid responsiveness. Although there was a trend of increasing response rate when there were more triggers used, the small sample size prevented it from reaching statistical significance. Moreover, there was no pattern of grouping these triggers that could achieve better fluid response. In post-surgical patients, Stephen reported that the combination of hemodynamic parameters and fluid balance history were better than any single parameter in predict low circulating volume measured by I125-albumin [23]. On the other hand, clinical parameters were found to poorly related to hemodynamic index in septic shock and ARDS patients [24]. Because the combination of FB triggers was not associated with higher response rate, it suggested they neither predict volume status nor fluid responsiveness.
Besides enrolling patients in late phase of septic shock, our study had some limitations. Firstly, many patients were transferred to our ICUs without fluid balance records made it impossible to calculate previous infused fluid. However, a rather high CVP before bolus suggested that they have received enough fluid in initial rescue phase. Secondly, considering patients safety, we only recruited patients with CVP ≥ 15 mmHg. Even so, the rate of response according to low CVP (≤ 8 mmHg) were not higher than other triggers made it very unlikely to be a better trigger if patients with higher CVP had been enrolled.
5. CONCLUSION
In optimization phase of septic shock, patients may still have indications for fluid administration, but fluid responsiveness is low, especially in patients already failed the initial bolus. No FB trigger was superior to the others in term of providing a higher responsiveness. Additionally, fluid responsiveness was not shown to be improved by the combination of these triggers.