1. INTRODUCTION
Perilla frutescens, commonly called perilla, is a common herb in many Asian countries including China, Japan, Korea... [2]. Fragrance in perilla leaves is due to the presence of essential oils including monoterpene derivatives, for example perillaldehyde, perillene, β-caryophyllene, limonene, perillaketone... [7]. Active chemical components such as ursolic acid and rosmarinic acid are also present in perilla leaves [15]. In addition, 16 flavonoids including 5 anthocyanidins, 2 flavones and 9 flavone glycosides were found in leaves and seeds of perilla. Among these flavonoids, 3-p-coumarylglucoside-5-glucoside of cyanidin and 7-caffeylglucosides of apigenin and luteolin were the major components [13]. Acid triterpene acid isolated from perilla leaves exhibited an anti-inflammatory effect on mice [15]. This effect was also exhibited by rosmarinic acid [4]. In a tumor carcinogenesis model triggered by DMBA and croton oil (or 12-0-tetradecanoyl phorbol-13-acetate, TPA), rosmarinic acid, luteolin, triterpene acid were proven to inhibit tumor formation and tumor progression [1, 3, 15].
The multi-stage skin carcinogenesis was one of the most commonly available and effective mouse models by using two different carcinogens, commonly DMBA and croton oil. After a single application of DMBA, an initiator of mutagen, repeated application of croton oil during a certain period of time, led to inflammation and stimulated tumor formation and progression.
In this study, we evaluated the antitumor-promoting effect of total perilla leaf extract 10% in mouse skin triggered by two-stage chemical carcinogenesis model, using curcumin 4% as a reference treatment.
2. MATERIALS AND METHOD
Perilla leaves, harvested at the age ranging from 50 to 60 days on December 2016 in Ha Noi (Vietnam) and authenticated by Prof. Thi Dep Tran, department of botany, Faculty of Pharmacy, University of Medicine and Pharmacy at Ho Chi Minh City, Viet Nam. Perilla leaves were dried, grounded in crude powder and extracted with ethanol 50 % under percolation protocol. The solvent was then evaporated at 95 oC until PLE humidity is lower than 20%.
Healthy male Swiss albino mice (25 - 30 gram) were purchased from Nha Trang Institute of Vaccine and Medical Biology, IVAC (Nha Trang, Vietnam). Mice were acclimatized to a 12-h light-dark cycle for at least one week before each experiment. The animals had free access to food pellets (IVAC, Nha Trang, Vietnam) and water ad libitum. 0ne day before the treatment, the dorsal skin of mice was shaved for an approximately 2 cm x 2 cm area. All experimental protocols were conducted under agreement of the scientific committee, specialty of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, University of Medicine and Pharmacy at Ho Chi Minh City, Viet Nam (Number 03-2016/QÐ-SÐHD).
DMBA (≥ 95%), croton oil and acid rosmarinic (≥ 98%) were purchased from Sigma - Aldrich Co. (Germany); curcumin (> 98%) was a gift from Domesco Pharmaceutical Co. (Dong Thap, Vietnam) and luteolin (≥ 95%) from Department of Pharmacognosy, Faculty of Pharmacy, University of Medicine and Pharmacy at Ho Chi Minh City (Ho Chi Minh City, Vietnam).
Humidity and total ash of PLE were determined in accordance with Vietnamese Pharmacopeia IV. The presence of flavonoid was determined by chemical reactions with FeCl3 10%, Na0H 10% and Mg/HCl.
The thin layer chromatography (TLC) was performed by using normal phase of silica gel F254 plate, mobile phase At the end of the 20th week, 50% of experimental mice consisted of toluene:ethyl-acetat:formic acid (5:4:1); 20 μl of each PLE/methanol, luteolin/methanol and rosmarinic acid/methanol were spotted. The chromatogram was allowed to dry and is then detected under UV 254 and 365 nm. It was also sprayed with a solution of vanillin-sulphuric acid to give colored compounds.
In HPLC procedure, a Shimadzu HPLC SPD-M20A and PDA detector were used to determine the contents of luteolin and rosmarinic acid in PLE. The quantitative analysis was performed on an Agilent Zorbaz C18 reverse - phase column (4.6 x 250 mm, 5 μm) at UV 300 nm at 25oC. The flow rate was 1.0 mL/min and the injection volume was 20 μl. The mobile phase consisted of acetonitrile and 0.1% phosphoric acid in water with ratio of 30:70. The percentage of luteolin and rosmarinic acid in PLE was expressed as mean ± SD.
The cutaneous tumors were initiated by a single application on the dorsal shaved skin 50 μl of a 0.2% DMBA solution prepared in acetone (equivalent to 100 μg DMBA per mouse). Two weeks later, 50 μl of a 2% croton oil solution prepared in acetone (equivalent to 1 mg per mouse) were topically applied two times weekly until the end of the experiment.
Mice were divided randomly into 5 groups. Group I (n = 6) was painted with 50 μl acetone on the dorsal shaved skin two times weekly. Group II to V were applied with DMBA/croton oil according to the carcinogenesis protocol. Group II (n = 9) served as carcinogen control group whereas group III to V were treated groups receiving topical application on the dorsal shaved skin once daily of glycerin 5% (group III, n = 14), PLE 10% (group IV, n = 16) and curcumin 4% in acetone ( group V, n = 9).
Body weight of mice, latency period of tumor formation, tumor incidence, and average tumor volume was observed and measured at weekly interval. An electronic digital caliper 110-WR Series was used to measure the width and length of the tumors and only those with dimension greater than 1 mm x 1 mm and persisted more than one week were taken into consideration for data analysis. Average tumor volume was measured using the formula [14]:
Where V is tumor volume, L and W are length and width of the tumors. Latency period of tumor formation was determined when the first tumor appeared. Tumor incidence was calculated by dividing the total number of tumor-bearing mice with the total living mice in a particular group. Tumor burden was obtained by dividing the total number of tumors with the total number of tumor-bearing mice in a group.
At the end of the 20th week, 50% of experimental mice were randomly selected by Microsoft Excel. Mice were then sacrificed and skin samples were isolated and fixed in 10% formalin. Tissues were embedded in paraffin wax, sectioned with microtome and stained with Haematoxylin and Eosin (H&E) stain. Stained slides were observed under light microscope for histopathological analysis using different magnifications. For general observation, 100x magnifications were applied to detect abnormal regions; for further analysis, 200x and 400x magnifications were used to classify the tumor progression degrees.
PLE were dissolved in glycerin 5% into concentration 10%. Group of 11 healthy mice were topically applied with 50 μl PLE 10% on dorsal shaved skin. The treatment was repeated daily in 20 weeks. At the end of experiments, mice were sacrificed and the dorsal skin was harvested for histological analysis.
SPSS 20 software and Microsoft Excel 2016 were used to calculate and analyze the data of experiment. SPSS 20’s statistical tests were performed including Shipiro-Wilk, Mann-Whitney, T-test, Chi-square and Fisher’s exact test. All values were expressed as mean ± S.E.M (standard error of means) at 5% significant level. Values with P < 0.05 were considered statistically significant.
3. RESULTS
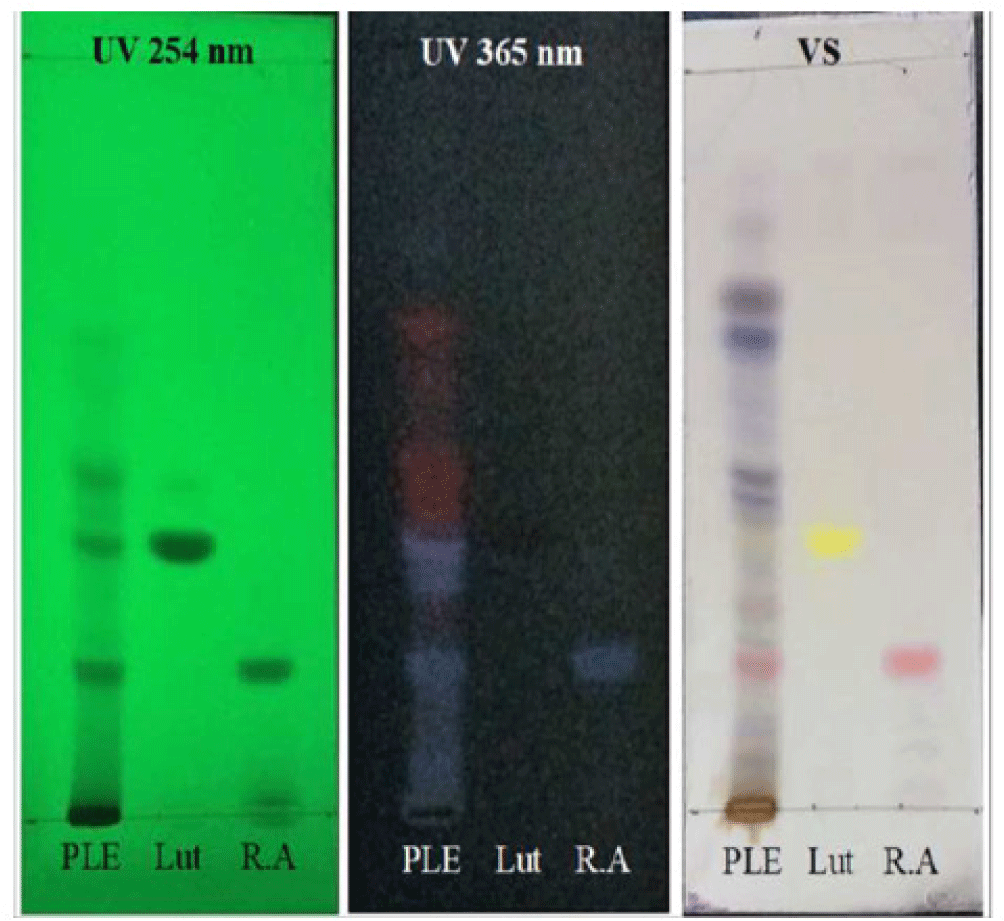
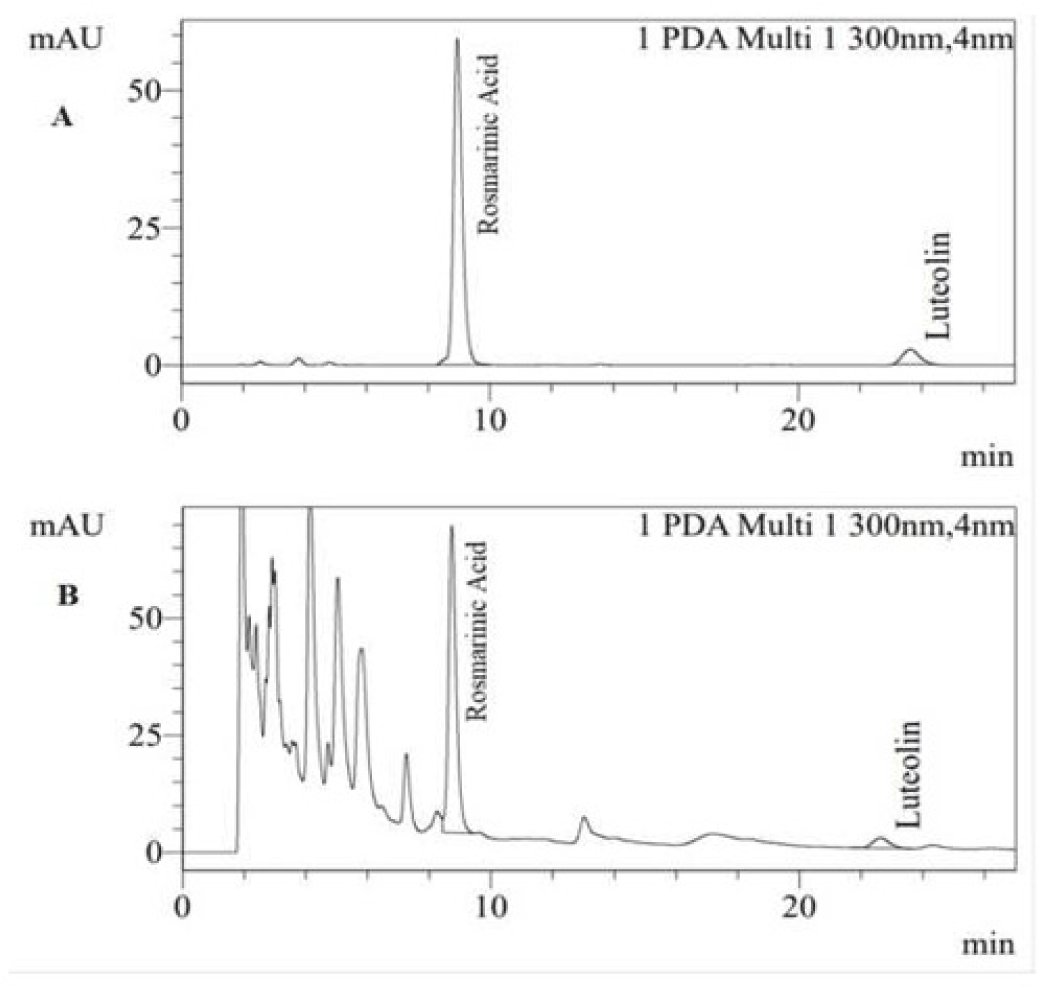
The mean humidity and total ash of PLE was 13.91% and 17.04%, respectively. The reactions to identify the presence of flavonoid were all positive to FeCl 10%, Na0H 10% and glycerin 5% group (Figure 3). The average tumor volume of Mg/HCl. The chromatogram showed that PLE spot appeared two marks having the same colors with rosmarinic acid and luteolin references with Rf values of 0.187 and 0.347, respectively (Figure 1). The result demonstrated the presence of these compounds in PLE. Moreover, these compounds were also found in HPLC chromatogram of PLE when comparing to luteolin and rosmarinic acid reference samples (Figure 2). The quantitative analysis showed estimated concentrations of rosmarinic acid and luteolin in PLE were 1.178 ± 0.011 and 0.105 ± 0.001% (w/w), respectively.
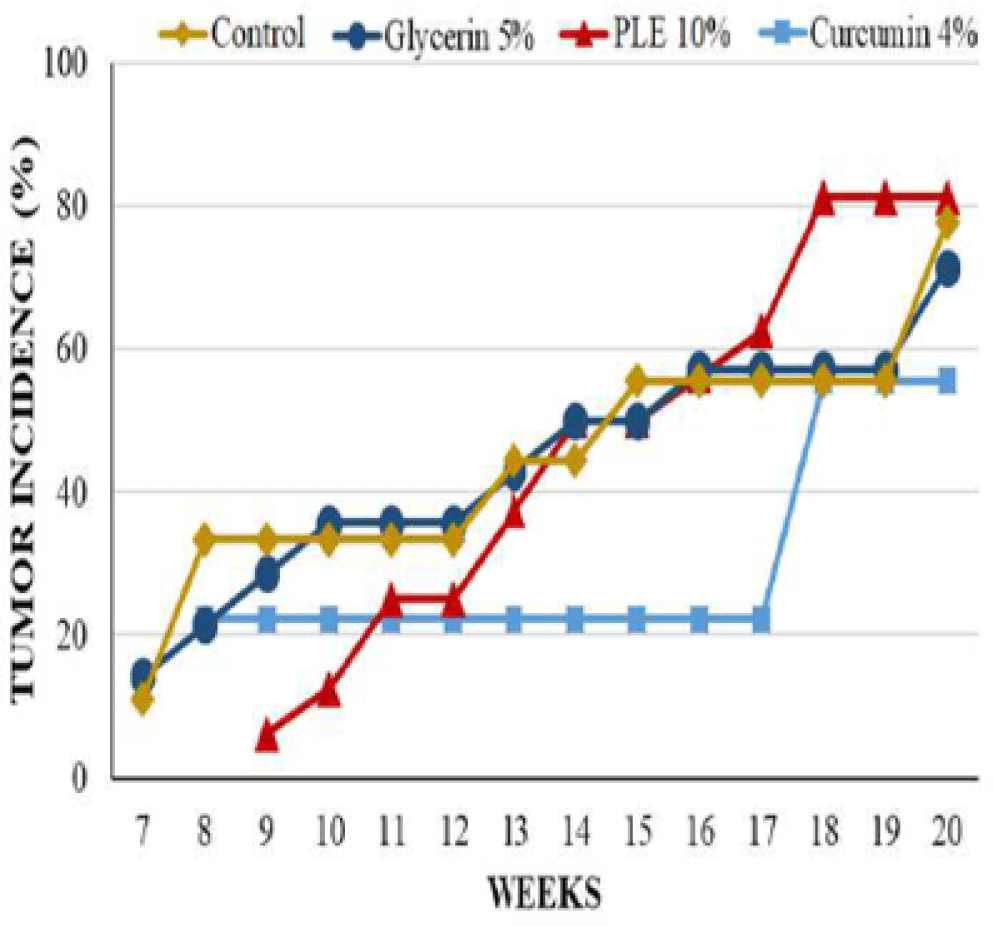
During 20 weeks of experiment, mice from acetone treated control group were tumor-free and had no significant abnormal phenomena appeared on the skin (Table 1 and Figure 7).
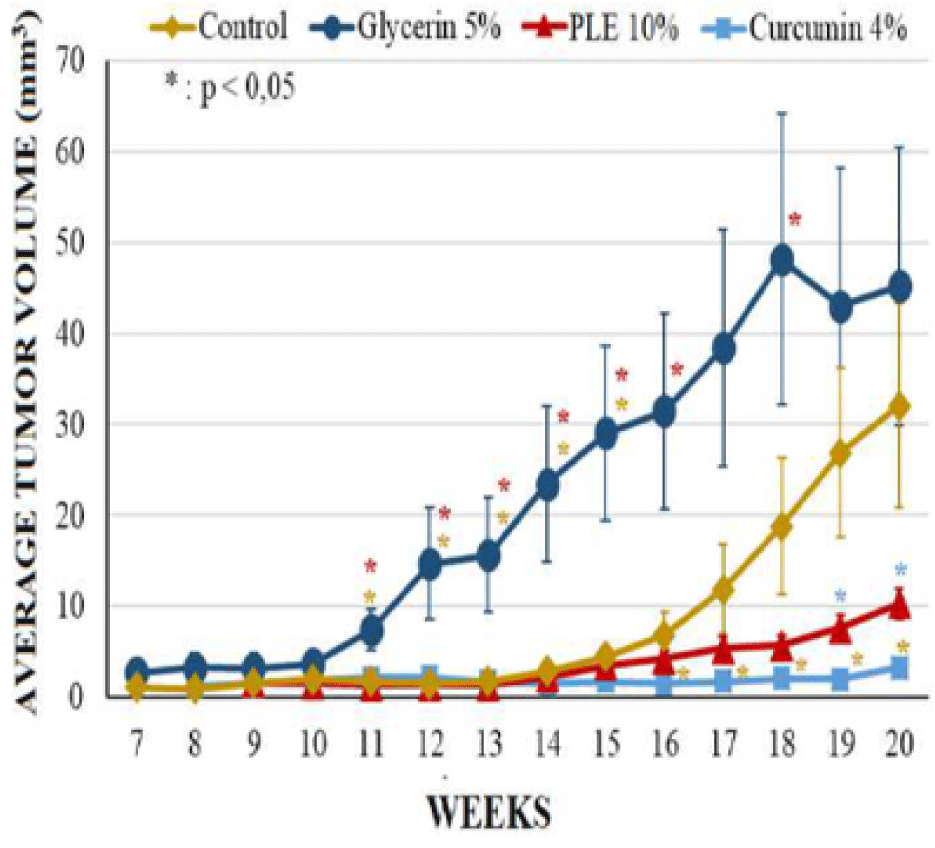
Mice from glycerin 5% group, however, had significant changes on morphology of dorsal skin. The tumor incidence as well as the tumor burden of this group were comparable to that of carcinogen control group at the termination of experiment; the first skin tumor appeared at week 7 on the mouse skin of these groups. However, the average tumor volume of glycerin 5% group was higher than control group during 20 weeks, especially tumors increased their size rapidly from week 11 to week 15 (Figure 5). At week 20, the average tumor volume of glycerin 5% group and control group was 45.26 ± 11.28 mm3 and 32.07 ± 11.25 mm3, respectively (Table 1).
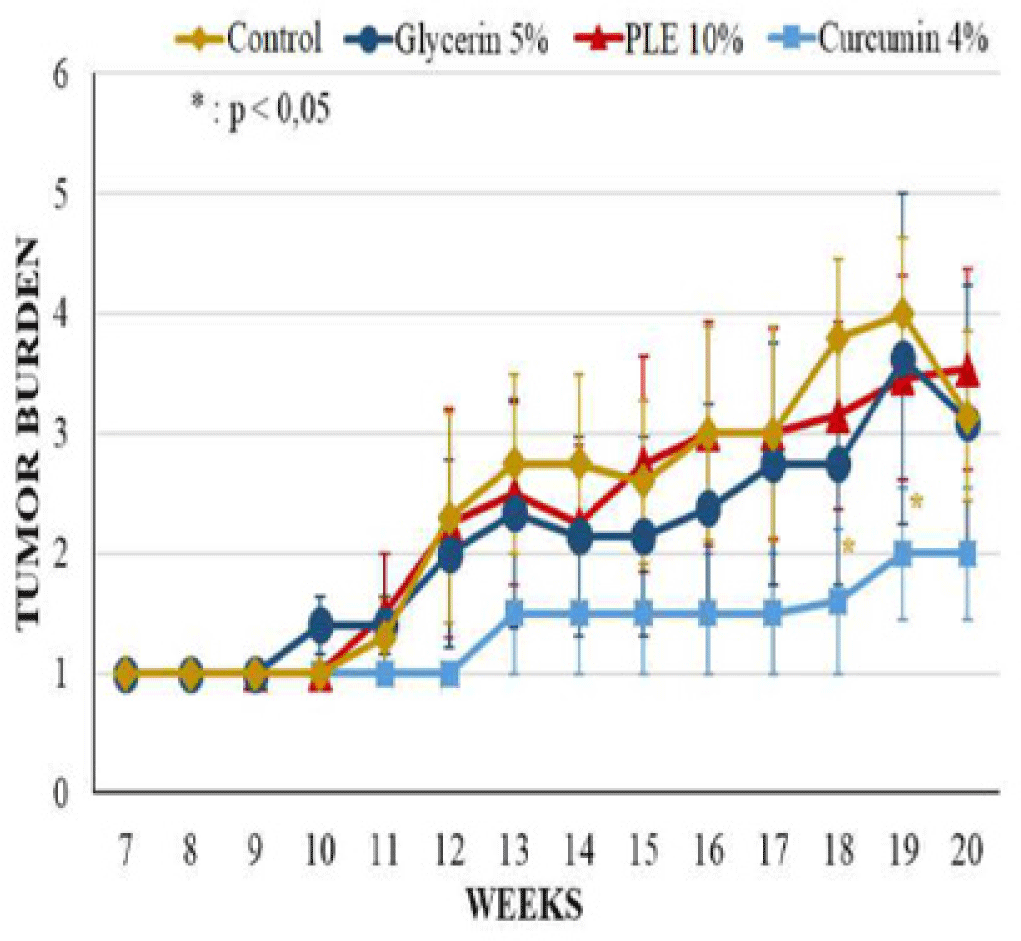
The latency period of tumor formation in PLE 10% treated group was greatly delayed to the ninth week. Curcumin 4% group developed the first tumor at week 8, sooner 1 week comparing to PLE 10% group but this group had the lowest tumor incidence and the lowest average tumor volume. 0n the contrary, the tumor incidence of PLE 10% treated group started continuously developing from week 13, leading to the last tumor incidence was slightly higher than this group increased continuously during 20 weeks but was much lower than glycerin 5% group. At the termination of experiment, the average tumor volume of PLE 10% group was only 22.76% of glycerin 5% group.
The differences between groups in tumor incidence and tumor burden had no significant statistical meaning (p > 0.05) (Figure 4). However, the average tumor volume of PLE 10% group was significantly different as compared to glycerin 5% group (p < 0,05) from week 11 to 16 and at week 18 (Figure 5). Compared to curcumin 4% group, PLE 10% group has higher average tumor volume significantly at week 19 and 20 (p < 0.05) (Figure 5).
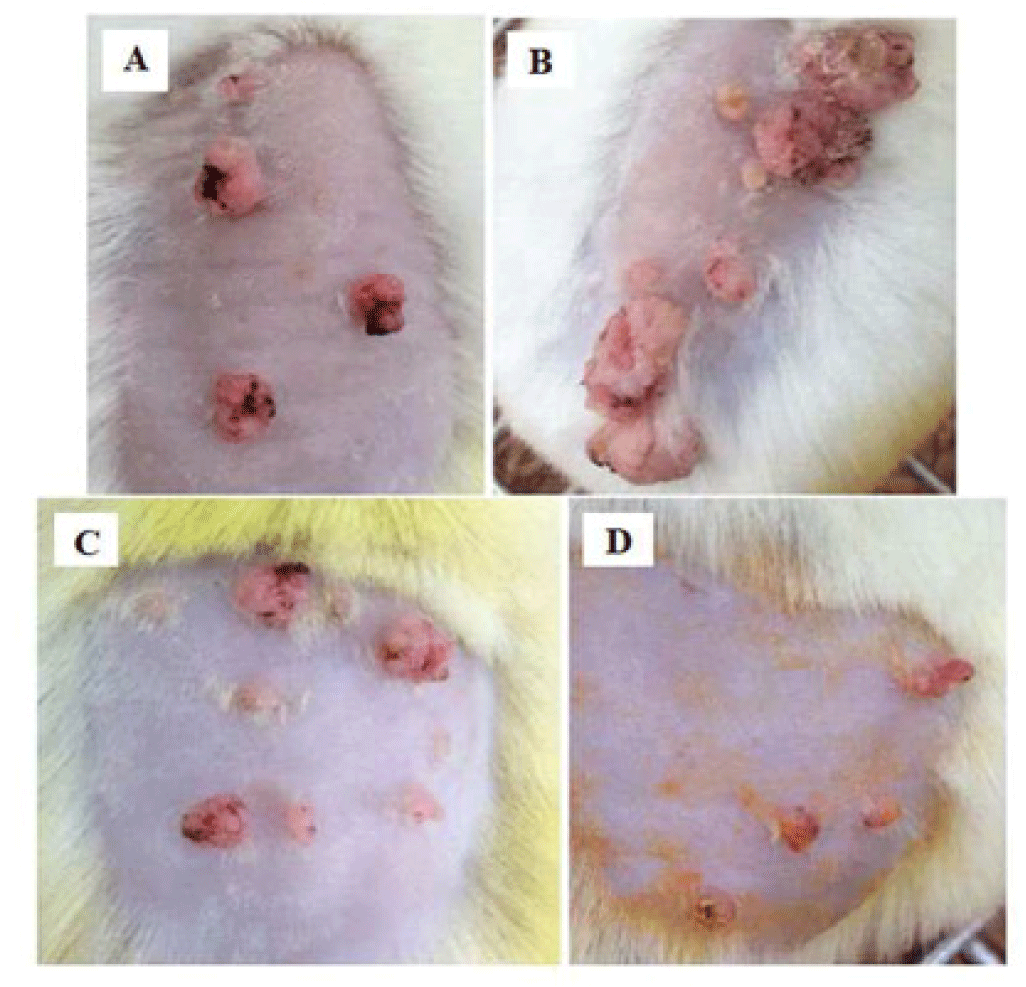
Morphological observation showed that the surface of tumors of PLE 10% group and curcumin 4% group appeared necrosis from week 14, later 3 weeks than groups of glycerin 5% and carcinogen control. Although PLE group had more number of tumors but these tumors were not as large as glycerin 5% group. The deformation of these tumors is also not severe as group of glycerin 5% (Figure 6).
The stained slides of mouse skin were observed under microscope to classify into three degrees of tumor progression. Hyperplasia was determined with feature of increasing thickness of stratum spinosum, granulosum and corneum. Papillomas with the composed mass of keratinizing squamous cells overlying a well-vascularized stroma. The diagnostic features of squamous cell carcinomas (SCCs) was characterized by the penetration of the mass and invasion of the dermis to the basal lamina [5].
The result presented in Table 2 showed that the percentage of hyperplasias from curcumin 4% groups and PLE 10% group higher than papillomas and squamous cell carcinomas (SCCs). PLE 10% group also had SCCs ratio as half of SCCs from glycerin 5% group. This demonstrated that the progression of these tumors was relatively slow in PLE 10% group. SCCs mice in all groups were well-differentiated type with keratin pearls and invasive growth into basement membrane. However, there were significant appearances of thickness hyperkeratosis and acanthosis of tumors from glycerin 5% and control groups (Figure 7).
| Groups | Hyperplasias (%) | Papillomas (%) | SCCs (%) |
|---|---|---|---|
| Control | 36.36 | 36.36 | 27.27 |
| Glycerin 5% | 57.14 | 14.29 | 28.57 |
| PLE 10% | 50.00 | 37.50 | 12.50 |
| Curcumin 4% | 63.64 | 18.18 | 18.18 |
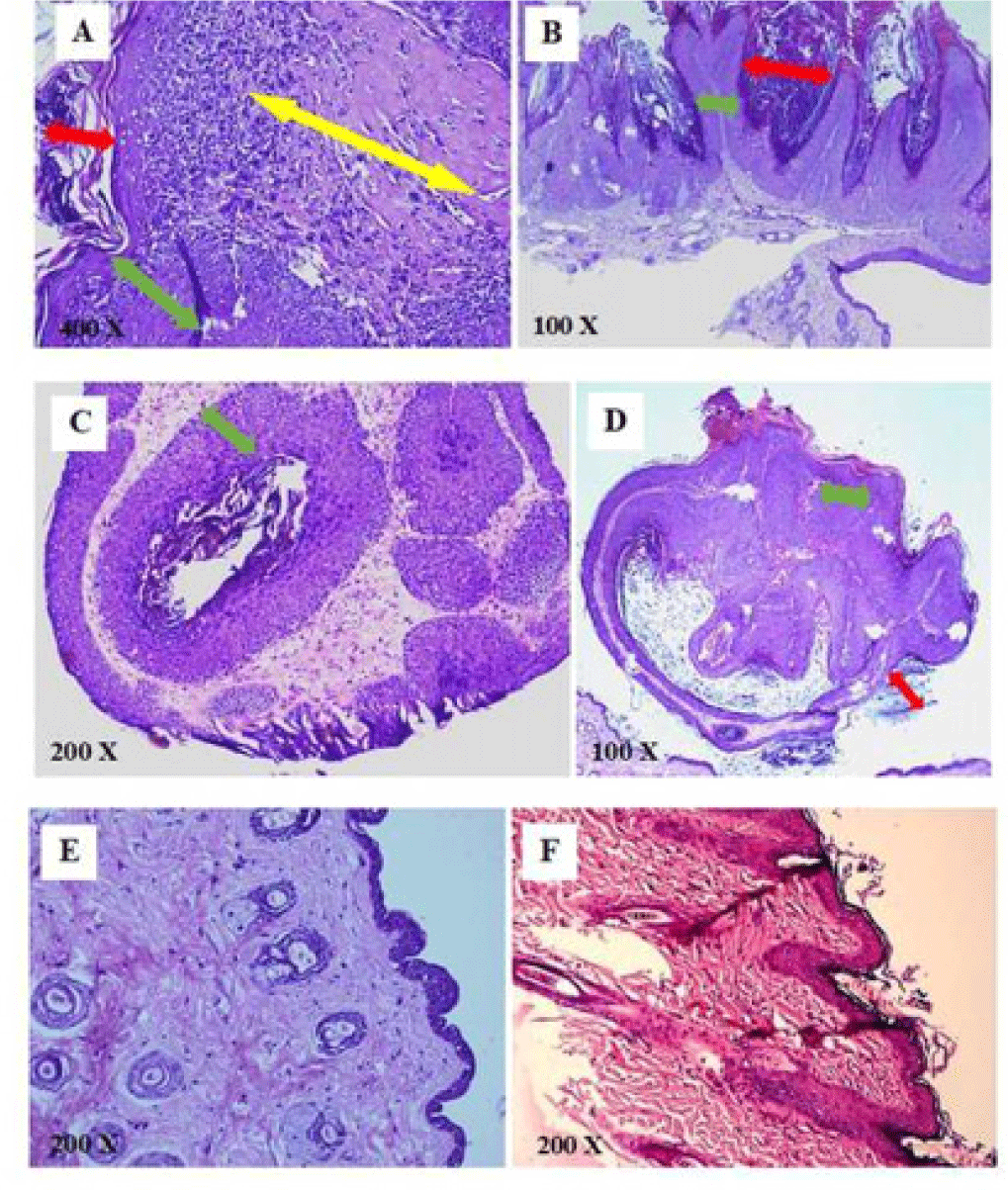
During 20 weeks of PLE applications, no noticeable modification on skin texture was observed in the PLE control group. The average body weight increased continuously about 2 times from the beginning to the termination of the experiment (26.13 ± 0.46 g and 50.51 ± 2.18 g, respectively). Moreover, no significant differences of epidermis layer were found on histological analysis (Figure 7).
4. DISCUSSION
The prevalence and risk of skin cancer have been increasing worldwide over past three decades [17]. Skin cancer mainly divides into two primary types: melanoma cancer and non-melanoma cancer (including basal cell carcinoma and squamous cell carcinoma). Melanoma cancer is rarely seen but malignant whereas non-melanoma cancer is more frequent, benign and clear response to treatment with low mortality in case of early detecting [16]. However, most skin cancers are diagnosed in late phase which requires appropriated surgeries to remove tumor tissues. The prevention of disease confers potential, effective and long-term measure in the war on cancer.
Among experimental models in animal which is mimic pathogenesis non-melanoma skin cancer, the two-stage carcinogenesis model was used extensively to investigate the anti-tumor effect of different compounds or extracts from medicinal materials. This protocol had the impact on observing initiation and promotion stages particularly, monitoring the influences of environmental factors or the effect of tested drugs on different stages of the tumor progression [12].
Recently, skin cancer chemoprevention is an attractive area for researchers to investigate by using phytochemical compounds to suppress or inhibit the process of carcinogenesis. Various natural compounds have been identified and proved as potential skin cancer chemo-preventive agents. In Perilla leaves, several flavonoids and triterpene acids demonstrated attracting pharmacologic activities such as anti-oxidant, anti-inflammatory and anti-tumorigenic effects [3, 5, 11]. Among these compounds, luteolin, as an important flavonoid in perilla proved to have anti-tumor effect, was chosen as a reference material of flavonoid identification [3]. Also, rosmarinic acid was the second marker thanks to its role as one of the major polyphenolic substances in P. frutescens species. The qualitative results showed that luteolin and rosmarinic acid both presented in perilla leaves, consistently with previous studies [9, 10].
The antitumor-promoting effect of extract from Perilla leaves has been confirmed by Ueda (2003) from in vivo experiments [3]. 0ral treatment with perilla extract showed no significant difference in tumor incidence but significantly lowered tumor volume of perilla-treated as compared to untreated groups. However, the extracting procedure, solvent dissolved extract, extract dosage and treating administration were different. In our study, total PLE was prepared in glycerin 5% as vehicle at the concentration 10%. Glycerin, as known as an unharmful chemical, was used as a moisturization element in many cosmetic products [8]. It was hoped to maintain PLE stayed on dorsal of mouse skin and intensify PLE permeability by softening the stratum corneum. However, the result showed that mice in glycerin 5% group although did not have differences of tumor incidence and tumor burden but it had significantly higher average tumor volume compared to the carcinogen control group. This might be due to the enhanced permeability and adhesion of the carcinogenesis agents thanks to glycerin properties. Topical application PLE 10% applied daily in 20 weeks did not reduce tumor incidence and tumor burden but prolong the tumor appearance two weeks and reduced significantly average tumor volume from week 14 to 16 and week 18 (p < 0.05), compared to glycerin 5% group. Histopathological analysis also showed that mice from group of PLE 10% performed the lower ratio of SCCs than glycerin 5% group. These results suggested that PLE helped to delay the tumor progression to SCCs. However, the effect was diminished in the terminal weeks, especially after week 14. This indicated that the antitumor-promoting effect was greatly in 14 weeks and decreased with the duration of treatment. Compared with curcumin 4%, as a refined anti-tumor substance, the effect of PLE 10% was quite lower.
5. CONCLUSION
The present study suggested the chemopreventive potential of topical application PLE 10% in skin carcinogenesis model induced by DMBA and croton oil. The effectiveness of PLE 10% though was not quite prominent, but the experiment has revealed the promising antitumor-promoting effect of perilla. Further investigations are needed to perform with the higher concentrations of PLE in order to better understand the detailed mechanism of the perilla leaf activities against the formation and progression of skin tumors.