1. Introduction of reactive oxygen species (ROS) and antioxidant systems
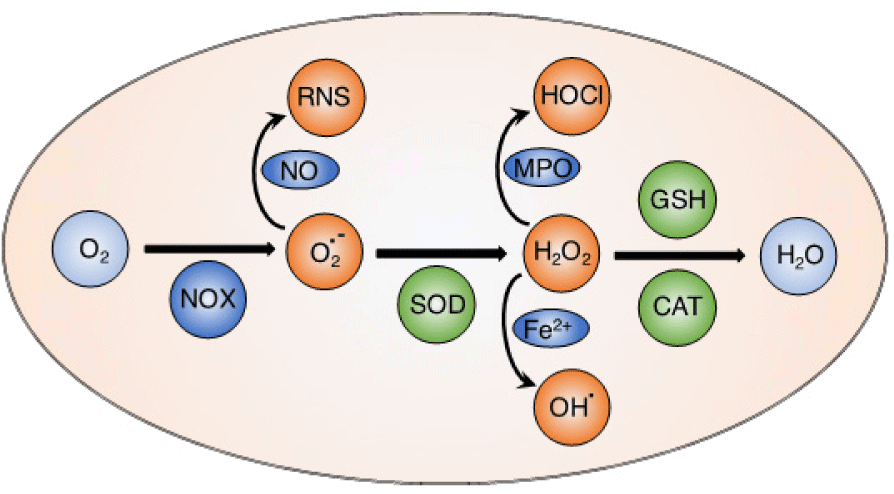
Reactive oxygen species (ROS) such as hydroxyl radicals (OH•), superoxide anion (O2•–), hydrogen peroxide (H2O2) and singlet oxygen (1O2) are highly reactive molecules containing oxygen. Mitochondria are well-known as an important source to generate ROS in most mammalian cells via electron transfer chain reactions [1, 2]. Basically, the oxygen molecules taken up during aerobic respiration and cellular metabolism are converted to water and carbon dioxide [3]. From the electron transfer chain, superoxide anion is generated via an electron transfer to oxygen molecule by NADPH oxidase (NOX). The superoxide anion is subsequently converted to hydrogen peroxide by superoxide dismutase (SOD). In the Fenton reaction, hydrogen peroxide is reduced to form hydroxyl radicals in the presence of Fe(II) ion. These electron transport reactions play critical roles to produce the cellular energy-carrying molecule, adenosine triphosphate (ATP). This physiological level of ROS is regulated by cellular antioxidant systems [4, 5]. Both ROS and antioxidants can be generated from endogenous and exogenous sources. Endogenous source of ROS includes respiratory chain in mitochondria as stated above, phagocytes, oxidase enzymes (xanthine oxidase, NOX, nitric oxide synthase.) and oxidized biomolecules (fatty acids, DNA, and protein), while exogenous ROS are generated by radiation (UV lights, X-rays, radioisotopes), pollution, dietary of toxic compounds, etc... On the other hand, endogenous antioxidants are mainly intracellular enzymes such as catalase, SOD, and glutathione peroxidase, while extracellular antioxidants can be found in body fluids like metal-binding proteins such as albumin, hemoglobin, myoglobin, cytoglobin, transferrin, lactoferrin, etc. In addition, exogenous antioxidants are provided from dietary (vitamins, polyphenolic, flavonoid compounds found in vegetables, green tea, wine, etc.). The right balance of generating ROS and antioxidant defense enzymes is critical to keep redox homeostasis in the normal physiological conditions [4].
2. Role of ROS in health
Under normal physiological conditions, ROS play critical functions as redox messengers for intracellular signaling and regulation (Figure 1) [4, 5]. Many important intracellular transcription factors and heat shock proteins are modulated by ROS to maintain redox homeostasis. In addition, ROS regulate ion transporters, cellular pH, and immune systems [6]. Several important endogenous antioxidants including glutathione, superoxide dismutase, and catalase contribute to maintain the balance between pro- and anti-oxidation consistently in the living organisms. Under inflammatory environments, ROS are generated by accumulating immune cells such as macrophages and neutrophils to react as a defense mechanism against the pathogens [7]. Generated ROS from this innate immunity prevents invasion of pathogens and inhibits their proliferation. In the acquired immune response, ROS are also involved to regulate and enhance the activation of the T lymphocytes [8, 9].
On the other hand, ROS are reported to induce the secondary antioxidant response via nuclear factor E2-related factor 2 (Nrf2), a critical transcription factor to regulate the antioxidant response element (ARE) in protecting cells from oxidative damage [10]. Basically, Keap1, a regulating cytosolic protein, forms the dimer with Nrf2 (Nrf2-Keap1) that promotes the ubiquitylation and degradation of Nrf2, resulting in inhibition of Nrf2 signaling. However, under oxidative stress condition or treatment with ROS such as hydrogen peroxide, the Nrf2-Keap1 dimer is not stable and Nrf2 is released from Keap1 to translocate from cytoplasm to nucleus, promoting the transcription of ARE-regulating genes, and the expression of antioxidant proteins and phase II detoxifying enzymes [11, 12]. ROS are also reported to regulate nuclear factor kappa B (NF- B), an important transcription factors in inflammation and immunity. Although several inhibitory effects have been reported, ROS are well- known to simulate the NF- B signaling, which is critically related to cellular development, differentiation, proliferation and cell survival [13].
3. ROS induce oxidative injuries and diseases
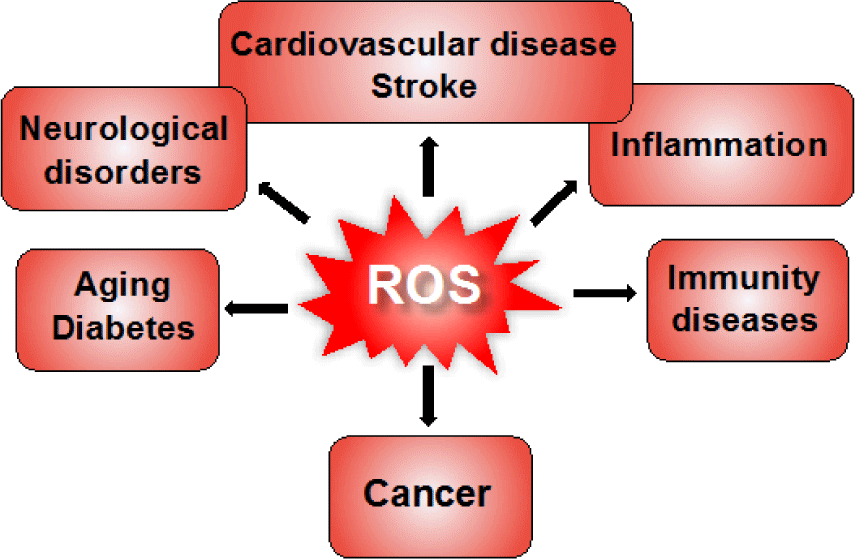
Although ROS can act as signaling molecules to maintain the intracellular redox equilibrium, under oxidative stress conditions, excessive generation of ROS causes oxidative damage to biomolecules including nucleic acids, lipids, and proteins (Figure 2) [14]. For example, hydroxyl radical directly reacts with DNA molecules, causing oxidative damage and leading to mutations in the DNA sequence. A highly reactive nucleotide, guanine, can rapidly interact with excess hydroxyl radicals to form 8-oxoguanine, which is known as a potential biomarker of mutagenesis. Carbonylation of protein molecules occurs during reaction of hydroxyl radicals with amino acids at the side chains, especially cysteine and methionine are particularly more susceptible to ROS. Free radicals can also attack directly the membrane lipid bilayer or polyunsaturated fatty acids in lipid molecules at the carbon-carbon double bonds, causing lipid peroxidation. Several products of lipid peroxidation are capable of inactivating many functional protein molecules inside the cells, leading to cellular dysfunction [15]. These self-sustaining cycles of oxidant productions may amplify inflammation, tissue injury, and subsequent cellular apoptosis. Therefore, ROS-mediated injuries are strongly related to numerous human diseases such as inflammation, stroke, myocardial infarction, diabetes, neurodegenerative diseases, aging, cancer, etc. (Figure 3) [16-19]. In fact, in patients with inflammatory bowel disease, the increase of ROS causes oxidative stress and oxidative cellular damage in the gastrointestinal mucosa, promoting carcinogenesis [20, 21]. During the intestinal injury, a signiicant iniltration of neutrophil and increase in myeloperoxidase (MPO) levels are observed in inflammatory sites. Generated ROS activate the key transcription factor NF-KB in the immune cells to accelerate the secretion of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and IL-6 [22, 23]. ROS have been reported for great contribution to neuronal loss in cerebral ischemia, Parkinson’s disease and Alzheimer’s disease [24]. It is interesting to notice that neural cells are considered more susceptible to oxidative damage as compared to other body tissues [24, 25]. Brains contains a high amount of fatty acid and ion, that may lead to the lipid peroxidation and generation of hydroxyl radicals, while antioxidant activity is lower as compared to other organs (about 10% of liver). In particular, excessive generation of ROS have been found in almost cancers, and it promotes the development, progression, and metastasis of tumor cells. As described above, excessive ROS rapidly interact with DNA to induce DNA damage and genomic instability, resulting in the abnormal growth of cells and mutagenesis. ROS have been widely reported to regulate several proliferation pathways in cancer cells. For examples, ROS activates the extracellular regulated kinase 1/2 in the mitogen-activated protein kinase pathway, increasing the cell proliferation. In IKK/NF-KB pathway, oxidative stress causes the degradation of IKB complex to translocate NF-KB into the nucleus, inducing the expression of anti-apoptosis and proliferation-related genes. In addition, ROS also regulate the PI3K/Akt pathway mediating cell survival [26-29]. Thus, excessive ROS promotes abnormal proliferation of cancer cells. On the other hand, many anticancer drugs induce ROS generation to kill cancer cells by direct necrosis or activating apoptosis via different pathways. When these anticancer drugs are utilized for a long time, however, it increases drug resistance to target cell and decreases their eficiency [30, 31]. In this way, ROS have been considered as a double-edged sword in ROS-mediated cancer therapies.

4. Consideration of antioxidant therapeutics for ROS- related diseases
Antioxidants have been used to counteract with the overproduced ROS for preventing and treating oxidative stress-related diseases. Several antioxidant drugs were approved for clinical use in various diseases such as edaravone for ischemic stroke, N-acetylcysteine for acetaminophen overdose-induced liver injury and α-lipoic acid for diabetic neuropathy [32]. In fact, there are 104 clinical trials of antioxidant therapies which are in phase 3 and phase 4 in total 462 trials [33]. However, numerous studies on using antioxidants as main therapy have shown controversial results in treatment of versatile diseases related to oxidative stress [32, 34-36]. Conventional antioxidants or ROS scavengers such as vitamin A, vitamin C, N-acetylcysteine, and glutathione have been used in several preclinical studies, which not always presented desired output in vivo, although their effectiveness was clearly shown in vitro conditions. Several antioxidants have been reported to be an effective treatment at the early stage of disease when ROS are initially generated to induce oxidative damage to biomolecules and tissue injury. However, antioxidants are not able to effectively recover the disease when they are used at later stage [32, 37]. ROS or oxidative stress may be not the only main factor that involved in the pathogenesis of disease. Combination of antioxidants with other drugs has shown the additive or synergistic eficacies [38-40]. It can also be explained that when these low-molecular-weight (LMW) antioxidants are administered, they spread non-speciically to the entire body and are rapidly metabolized, leading to low bioavailability and low activity at the target sites. In addition, LMW antioxidants facilely internalize into the healthy cells and may interrupt the important electron transfer reactions in mitochondria, leading to unwanted adverse effects [32, 41].
Therefore, improvements of bioavailability, stability and diseased speciicity of antioxidants need to be addressed to achieve the higher antioxidant therapeutic eficacy.
5. Current approaches for effective antioxidant therapies
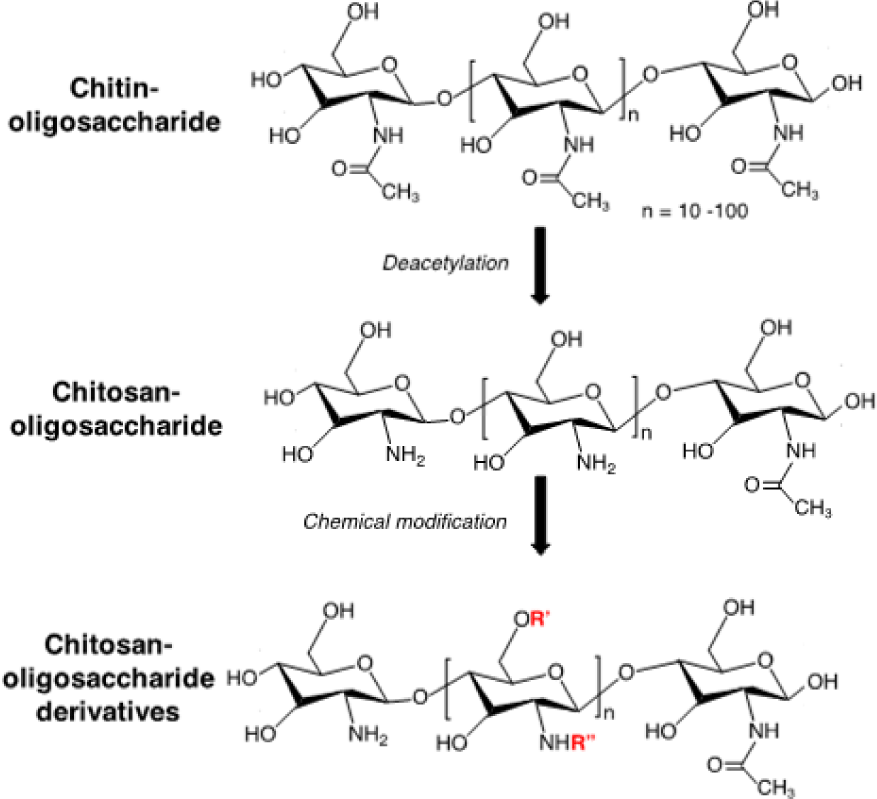
The research to develop novel antioxidant compounds is one of the most promising approaches to optimize the therapeutics for ROS-induced tissue injuries. The use of natural antioxidants has gained attention worldwide due to their presumed safety with nutritional and therapeutic values. For many years, our group has investigated and studied the antioxidant activities of compounds from nature, in particular chitin and chitosan. Chitosan is a biodegradable and biocompatible polymer prepared by alkaline deacetylation of chitin, which is a major component of exoskeletons of marine crustaceans (Figure 4). Chitosan and chitin have numerous biological activities such as immuno-enhancing activity, antitumor activity [42], antibacterial activity [43], antifungal activity [44], anti-hypertensive effects, and antioxidant activity [45, 46]. However, these biological activities are signiicantly limited since they are poor water soluble. To improve water solubility and biological activities of chitin and chitosan, chitin-oligosaccharides and chito-oligosaccharides have been prepared with different molecular weights, which are obtained by the chemical or enzymatic hydrolysis of chitin and chitosan, respectively. These oligosaccharides exhibit low cytotoxicity and high water soluble property, promoting their use in vitro and in vivo. Chitin-oligosaccharide and chito-oligosaccharide showed a signiicant increase in antioxidant activity against oxidative stress in mouse macrophage cells as compared to chitin and chitosan [47, 48]. Because polymer backbone of chitosan possesses primary amine and many hydroxyl functional groups, which are facile for chemical modiication, different derivatives of oligosaccharide such as alkyl-chitooligosaccharides, aryl- chitooligomers, gallyl-chitooligosaccharides, carboxylated- chitooligosaccharides, sulfated-chitooligosaccharides have been synthesized and investigated for biological activities (Figure 4). Interestingly, these derivatives of oligosaccharide also exhibited remarkably higher antioxidant activities as compared to original oligosaccharides [49, 51]. On the other hand, these derivatives possessed different bioactivities such as inhibition of angiotensin-converting enzyme and anti- inflammatory actions [52, 53]. These results indicated that chitin-, chito-oligosaccharides and their derivatives can be used as a scavenger against oxidative stress through control of the radical induced damage to cellular systems and show promise for other applications in biomedical fields.
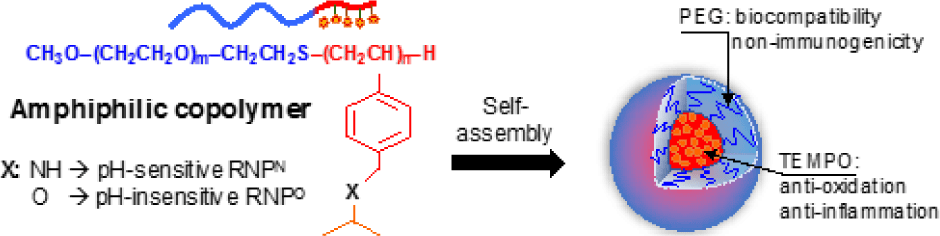
One of the other strategies to improve antioxidant therapeutics is to apply nanotechnology in medicine, so- called nanomedicine. Nanoparticles used in medicine such as liposome, polymeric micelles, dendrimers, and metal nanoparticles have attracted signiicant interest in medical treatment because they can speciically deliver a high amount of therapeutic agents to targeted diseased tissues [54, 55]. For examples, after systemic administration, nano-sized macromolecules tend to accumulate in the tumor microenvironment due to the increased vascular permeability at tumor tissue or enhanced permeability and retention (so called EPR) effect [56-59]. At the present time, the number of nanoparticles in the medical application was studied in preclinical and clinical settings as new diagnostic tools and therapeutic developments. Several nanomedicines such as have been clinically used for the treatment of human diseases, particularly cancer therapy, to overcome the poor bioavailability and severe side effects of conventional drugs such as paclitaxel and doxorubicine. Doxil , PEGylated liposome loaded with doxorubine, is the irst FDA-approved nano-drug in 1995, while paclitaxel albumin-bound nanoparticle (Abraxane®) has been approved for treatment of breast and lung cancers [60-62], indicating a promising approach for future medication. Recently, our group is also interested in stable nitroxide radicals such as 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO) because their molecular structures are able to react as a strong antioxidant to counteract with other free radicals. In addition, nitroxide radicals possess an unpaired electron, which can be monitored by electron spin resonance as a biophysical tool. For biomedical application, these nitroxide radicals have been studied in radioprotection, functional imaging, antioxidant and anticancer treatments [63, 64]. Under physiological environments, however, treatment of these LMW nitroxide radicals exhibits low therapeutic window because of many issues including preferential renal clearance, rapid metabolism to the low activity hydroxylamine form, and non-speciic distribution to normal tissues, leading to adverse effects. To overcome such issues, we have recently developed novel redox nanoparticles (RNPs), prepared by an amphiphilic block copolymer containing stable nitroxide radicals in the hydrophobic segment as a side chain. Nitroxide radicals can be covalently conjugated to the hydrophobic chain via an amine linkage to form nanoparticle with pH- sensitive character (RNPN) or via ether linkage to form the nanoparticle with pH-insensitive character (RNPO), as shown in Figure 5. By simply self-assembling in the physiological environment, amphiphilic copolymer forms the core-shell- type micelle structure with a diameter of approximately several tens of nanometers, in which nitroxide radicals are stabilized in the core (Figure 5) [56, 65]. For several years of developments, we have investigated the antioxidant therapy of RNPs in oxidative stress injuries models in mice such as intestinal inflammation [66, 67], renal failure [68], liver injury [69, 70], neurodegenerative diseases [71, 72], and cancer [73]. Our results showed that RNPs presented a signiicantly higher therapeutic eficacy in these diseased mouse models as compared to LMW nitroxide radicals. It is interesting that the combination of RNPs with conventional chemotherapies such as doxorubicin, irinotecan, and pioglitazone showed the synergistic eficacy in several cancer model mice, with minimizing the adverse effects of these chemotherapies [74-76]. RNPs not only could improve the bioavailability of conventional antioxidants but also could suppress the oxidative degradation of antioxidant such as curcumin [77]. As we described above, most of LMW antioxidants present a low bioavailability, instability in vivo condition, and non- speciic distribution after administration to the body. We wanted to emphasize that by modulating the antioxidant to nanoparticles formulations, we could speciically deliver the antioxidants to diseased tissues, but not in normal tissues, which is important to enhance targeting therapy, and suppress unwanted side effect of antioxidant [78]. In fact, we have investigated that RNPs showed much lower toxicity to normal cells, zebra ish embryos, and mice models even in long-term treatment as compared to LMW nitroxide radicals [36, 56, 64].
Many other strategies have been developed to improve antioxidant eficacy and showed promising results. These applications of conventional and novel antioxidants have shown therapeutic effects in vitro and in vivo; however, it is still dificult to pass the preclinical and clinical settings due to the differences of cell/animal research from human trial and complicities of disorders. In summary, regulation of ROS and antioxidant systems to maintain the intracellular redox equilibrium is a key challenge for scientists and medical doctors in the treatment of ROS-related diseases.