1. INTRODUCTION
The introduction of non-Vitamin K oral antagonist anticoagulants (NOACs) was revolutionary in clinical practice in many aspects since they directly impact the coagulation pathway, are less likely to interact with foods or other medications, and with predictable anticoagulant effects of the NOACs. Dabigatran etexilate (dabigatran), has many benefits in the prevention of stroke or systemic thromboembolism caused by non-valvular atrial fibrillation (AF) and in the reduction of bleeding complications compared to vitamin K antagonists [1]. Before the introduction of idarucizumab, the treatment of dabigatran-induced bleeding included supportive strategies and symptomatic treatment [2]. The result from the RE-VERSE AD trial showed that idarucizumab, a monoclonal antibody, can be successfully administered to rapidly reverse the anticoagulant effects of dabigatran for patients with life-threatening haemorrhage or those who required urgent procedures [3-4]. However, clinical experience in using idarucizumab for anticoagulation reverse in urgent/emergency procedures or drug-induced major bleeding is limited in Asian patients, especially Vietnam [5].
2. CASE REPORTS
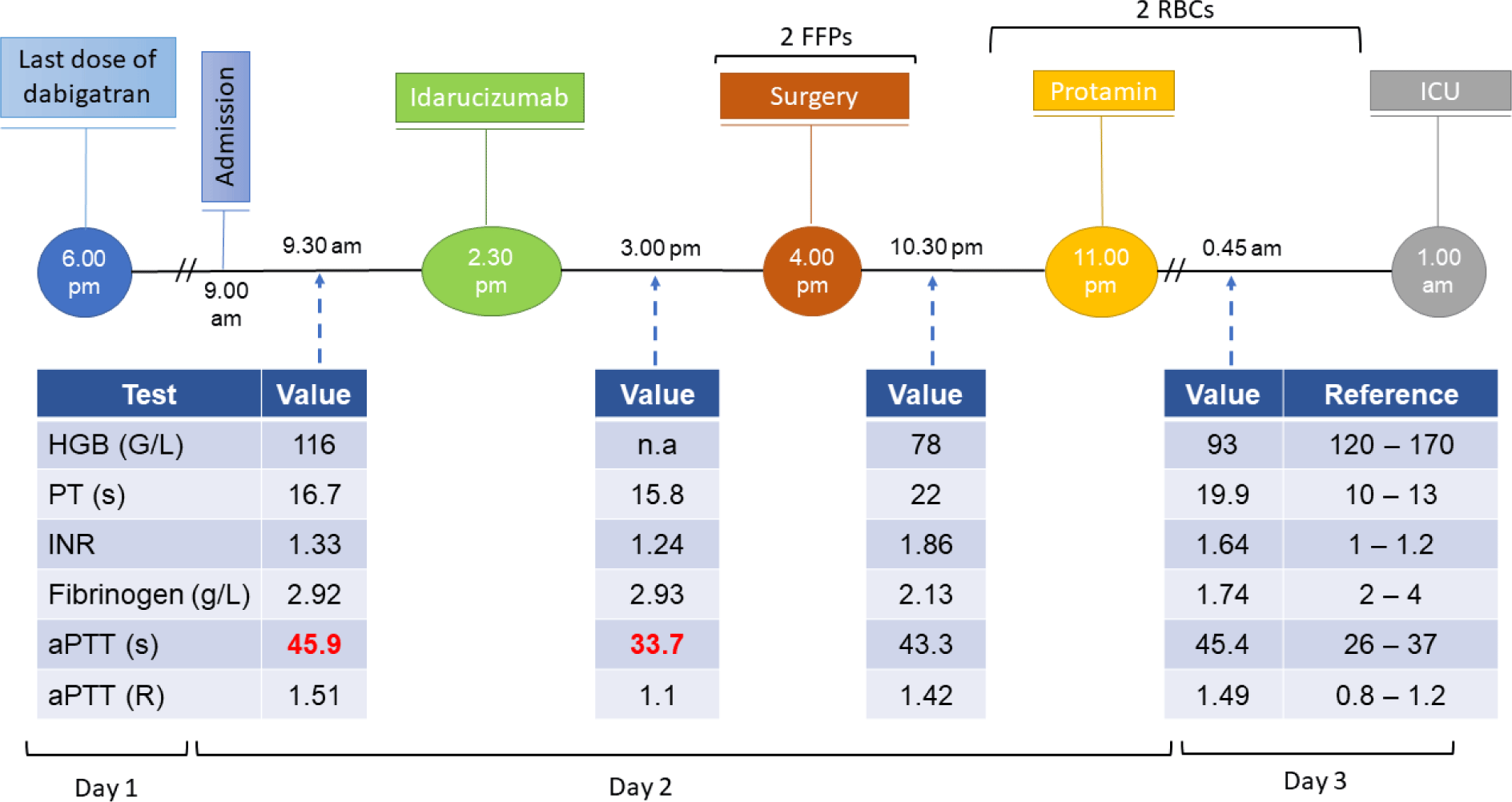
A 61-year-old male patient with a 41-year history of dilated cardiomyopathy, on the waiting list for heart transplantation, and chronic AF, presented to an outside hospital with a chief complaint of shortness of breath, which was identified as an acute exacerbation of chronic heart failure. The patient’s symptoms were successfully reduced with dobutamine (later switched to digoxin), furosemide, tolvaptan, sacubitril/valsartan and spironolactone. Dabigatran was used for prevention of ischemic stroke associated with chronic AF. Shortly after symptom improvement, the patient was transferred to Cho Ray Hospital for heart transplantation. A matched donor’s heart was successfully procured for the patient. Due to the time constraints for transplantation of the donor’s heart, the patient required immediate anticoagulant reversal. His coagulation status was abnormal on admission reflecting the effects of dabigatran, with a PT (prothrombin time) of 16.7 seconds (reference range: 10 – 13); an INR (International Normalized Ratio) of 1.33; an activated prothrombin time (aPTT) of 45.9 seconds (reference range: 26 – 37); and an estimated glomerular filtration rate (eGFR) of 55 ml/min/1.73m2 (reference value > 90).
The patient was given idarucizumab 5 grams in 2 bottles (about 10 minutes apart). After 30 minutes, his coagulation status was approximately within range normal: PT was 15.8 seconds, INR was 1.24, and aPTT was 33.7 seconds. Heart transplantation procedure started 1.30 hours after idarucizumad administration and took 7 hours to complete. Heparin was used during the procedure and protamine sulfate was given postoperatively, guided by aPTT levels. 700 mL of red blood products derived from whole blood was administered postoperatively to correct blood loss during the procedure. Total blood products administered during cardiopulmonary bypass was measured to be 400 mL of fresh frozen plasma and 700 mL of packed red blood cells. The clinical course for this patient is shown in Figure 1. He was discharged after 40 days with significant improvement of symptoms.
A man was 53 years old with medical history of chronic AF taking dabigatran 150 mg twice a day, presented to the emergency department for episodes of waxing and waning abdominal pain for 1 day. He first experienced the pain while at rest, which lasted for 15 minutes and then recurred multiple times throughout the day. The episodes became worse and the pain had migrated from the epigastric region to the right iliac region, at which time he presented to the emergency department. Upon initial examination, the patient was awake and conscious, with a blood pressure of 120/60 mmHg, and an irregularly irregular heart rhythm of 80 beats per minute. The abdominal examination demonstrated tenderness to palpitation of the right iliac region. Other examinations were normal. His CHA2DS2-VASC score was 1 point and his HAS-BLED score was 2 points.
Laboratory tests on admission showed elevated creatinine concentration of 1.73 mg%, normal PT and prolonged aPTT (Table 1). An emergency abdominal computed tomography (CT) showed an enlarged appendix filled with fluid and infiltrated tissue. The patient had been diagnosed with acute appendicitis and an emergency laparoscopic appendectomy was scheduled.
The last dose of dabigatran was administered 1 hour and 20 minutes prior to admission. Therefore, idarucizumab (Praxbind), 5g was administered as a single intravenous bolus. The patient then underwent the appendectomy; the surgery was performed in 1 hour and 30 minutes without complications. No major or minor bleeding was noted, and no blood transfusion was needed. His postoperative coagulation panel showed significantly reduced PT, aPTT, and complete normalization of coagulation parameters. At follow-up 1 day and 3 days post-surgery, the patient was stable with resolved abdominal pain. To reduce the risk of stroke, dabigatran was reinitiated at the dose of 110 mg twice a day, 2 days following surgery. He was discharged 3 days after surgery with no signs of bleeding.
A 90-year-old female with a history AF was admitted following a fall with acute head and loss of consciousness. She denied history of stroke, seizures, and any head injuries. Her son reported that she was taking dabigatran 110 mg twice a day for the prevention of ischemic stroke associated with AF. In the emergency department, she was conscious and oriented, and general physical examination was unremarkable with normal vital signs. Glasgow Coma Scale (GCS) was 15/15 with no focal sign of neurologic deficits. There was no other signs of external trauma except for mild bruising over the left temporal area. Her blood pressure was 140/85 mmHg, with an irregular heart rate of 90 beats per minute. At presentation, her aPTT was 72.2 seconds, PT was 14.3 seconds, INR was 1.34, hemoglobin was 126 G/L, and eGFR was 43 ml/minute/1.73 m2. Non-contrast head computed tomography (CT) revealed a mild focal left temporal subarachnoid haemorrhage without any midline shift and a left temporal fracture. Her neurological status was monitored closely.
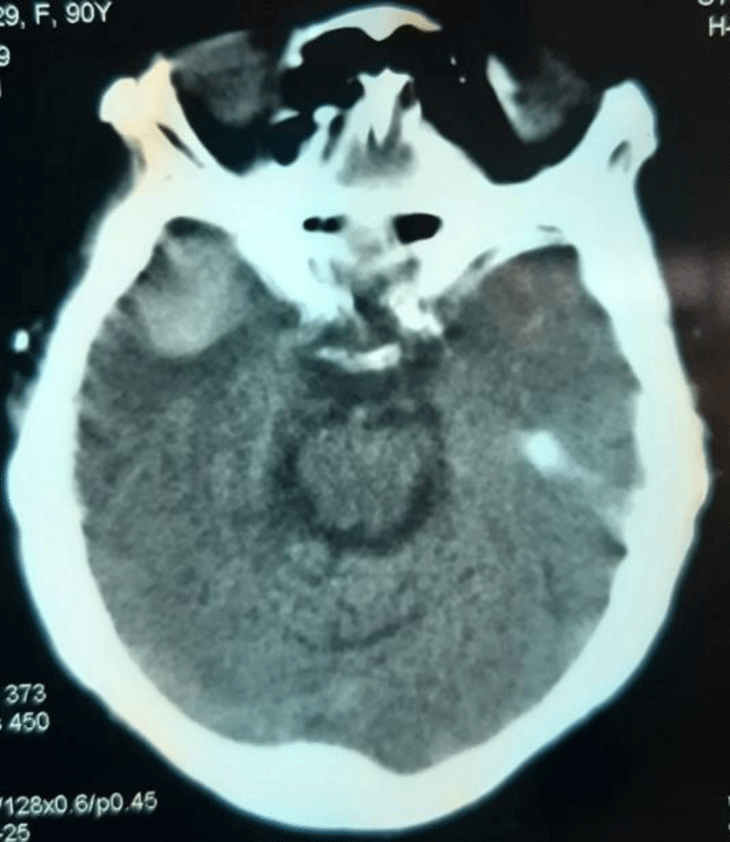
After 8 hours, her mental status progressively deteriorated, she had recurrent vomiting and became agitated. Her speech became difficult and GCS was 13/15 (E3V5M5). Immediately, she underwent a second head CT that showed a new intraparenchymal haemorrhage in the right temporal lobe and increased severity of the subarachnoid haemorrhage in the left parietal region, without any space-occupying effect (Figure 2). She was given with 2 bottles of idarucizumab containing 5 g by intravenous over 20 minutes. After idarucizumab administration, her PT was 15.5 seconds, INR was 1.15, and aPTT was 33.1 seconds. Over the next 24 hours, her consciousness improved, her vomiting was reduced, and her GCS stabilized at 14 points. Repeat head CT did not reveal any change in the size of hematoma. She was subsequently discharged on hospital day 7 with scheduled follow up visit.
3. DISCUSSION
We report here 3 cases of idarucizumab administration in our hospital, a large urban medical center. In 2010, dabigatran is a novel oral anticoagulant licensed by the FDA for prevention of systemic thromboembolism or ischemic stroke in patients with non-valvular AF. The indication of dabigatran as the first-line drug in NOAC-eligible patients with non-valvular AF received a class A of the recommendation from the ACC/AHA guideline in 2019 [6]. All of patients were prescribed dabigatran therapy for non-valvular AF.
These cases offer a cross-section view of real-world application of idarucizumab. In our hospital, when idarucizumab is indicated, we follow the same protocol used in the RE-VERSE AD trial, a multi-center, prospective, single cohort study [3]. Among the 503 patients in this trial who were taking dabigatran, who had life-threatening or uncontrolled bleeding (group A) or in need of emergency/urgent operations (group B), and had abnormal hemostasis at baseline, idarucizumab inverted anticoagulation rapidly and completely in more than 98% of the study population in the trial. A 5-g idarucizumab dose was effective in 98% of the study population. The reversal effect was well-kept during 24 hours in most patients. The first two patients were in group B. We reported a use of idarucizumab during an urgent cardiac transplantation in a patient with diagnosis of the end-stage heart failure and AF taking dabigatran. A heart transplant is a major procedure with a potential risk of life-threatening bleeding. Dabigatran was administered prior to cardiac transplantation for prevention of stroke or systemic thromboembolism in non-valvular AF. In case 2, the patient had acute appendicitis requiring an emergency surgical appendectomy. Understandably, rapidly reversing the effect of NOACs is necessary to reduce the occurrence of major bleeding during an emergent surgery. In case 3, the patient suffered from an intracranial hemorrhage cause by head trauma while taking dabigatran. Notably, traumatic brain injury is the main cause of acute subdural hematoma in the elderly population [7]. Delayed intracranial hemorrhage with NOACs and head trauma had also been described in elderly patients [8]. Unfortunately, our patient had delayed intracranial hemorrhage and her clinical condition progressively deteriorated. This patient met the group A criteria for administration of idarucizumab with life-threatening bleeding.
Dabigatran has the maximum anticoagulant effect within 2-3 hours after administration. It is renally cleared, and the half-life is approximately 12 hours, and its half-life can be prolonged in patients with an impaired kidney function [9]. It is consistent with the inclusion criteria of the REVERSE-AD study, that physicians choiced patients for idarucizumab without evaluate to diluted thrombin time or ecarin clotting time values that were predictive of the dabigatran’s anticoagulant effect [3]. At our hospital, these tests were unavailable, only PT and aPTT levels are routinely monitored. Although the dabigatran levels in blood does not correlate with aPTT, a prolonged aPTT is helpful in predicting the dabigatran’s anticoagulant effects [10]. The decision to use idarucizumab in these patients depended on time of the last dose of dabigatran, clinical condition, and evaluation of bleeding condition. The average patient-recorded time from the last time taking of dabigatran to idarucizumab administration was 7.5 hours in the first two patients and 10 hours in the third. There was a significant reduction in aPTT in all 3 cases after idarucizumab administration (Table 1). The baseline characteristics of the 2 patients with moderately impaired renal function (eGFR < 60 ml/min/1.73 m2) and in 1 patient with acute kidney injury are shown in Table 1. Dose adjustment in patients with reduced kidney function is not required [11]. Regardless of prior kidney function, reversing the dabigatran’s anticoagulant effects is with idarucizumad still safe and effective [12].
All 3 of our patients had a good clinical outcome after idarucizumab administration. The time to the beginning of the surgery in case 1 and case 2 was 1.6 hours and 4 hours, respectively. Normal hemostasis was recorded in these patients. During the operation, there were no bleeding events or any other associated events. In the RE-VERSE AD trial, the median time from the administration of idarucizumab to the initiation of the procedure was 1.6 hours, and most patients (95%) had either normal or mildly abnormal hemostasis during the operation [3]. In case 3, after use of idarucizumab, the patient’s condition gradually stabilized, and she did not require a craniotomy. Idarucizumab administration in patients with intracranial haemorrhage can provide a more effective strategy to neutralize the anticoagulant effects of dabigatran compared to alternative strategies [13]. Therefore, the patient received idarucizumab with successful reversion of anticoagulation effects of dabigatran. Then, she was treated with conservative management.
In April 2018, following the positive results from the RE-VERSE AD trial, idarucizumab (sold under the brand name PRAXBIND) was approved fully by the FDA as a reversal agent for dabigatran in the situation of emergency/urgent procedures or in life-threatening or uncontrolled major bleeding [14]. To the best of our knowledge, these are the first three patients given idarucizumab for reversing dabigatran’s anticoagulant effects in Vietnam successfully. The highly efficacious reversal we presented here was consistent with the published results from the RE-VERSE AD trial, which also included patients requiring urgent interventions or life-threatening situations while anticoagulated with dabigatran.
4. CONCLUSION
The use of idarucizumab can be considered as a more effective approach for patients with an urgent indications for reversing the anticoagulant effects of dabigatran. Idarucizumab here helped normalize activated prothrombin time and facilitated both an emergency surgery, as well as management of life-threatening bleeding regardless of renal function in our patients.
