1. INTRODUCTION
Secondary infertility is defined as unable to get a clinical pregnancy after one year of unprotected sexual intercourse in couples with a history of at least one pregnancy, birth, miscarriage, planned abortion, or ectopic pregnancy [1]. Infertility impacts on financial burden on patients and the health care system as well as in psychological aspect for millions of couples [2]. Among the major causes of female infertility, endocrine or ovulatory dysfunction together with uterine or peritoneal diseases are most common, whereas tubal disorder is the second common causes of secondary infertility in women [3].
Pelvic inflammatory disease (PID) is one of the most important causes of tubal disorder leading to infertility. Beside Neisseria gonorrhea and Chlamydia trachomatis playing as the two mostly frequently associated to upper genital tract infections [4], the female genital tract is an appropriate environment for the growth of other pathogenic and non-pathogenic microorganisms. Mycoplasma is a bacterium that can cause asymptomatic to minimally symptomatic genital tract infections but can result in chronic complications, including infertility. Previous published studies have shown that Ureaplasma urealyticum and Mycoplasma genitalium are responsible for lower genital tract infections (vulvovaginitis) as well as upper genital tract infections such as endometritis and pelvic inflammatory disease [5-7]. M. genitalium was detected by swab from the cervix in 19.6% of all infertile and in 4.4% of fertile women [7]. Furthermore, the pregnancy rate after elimination of U. urealyticum was significant improved [5]. Pelvic inflammatory disease caused by these bacteria can induce tubal disorders of varying degrees, including tubal occlusion or hydrosalpinx.
U. urealyticum primarily colonizes in the human urogenital tract and in the vaginal flora but it does not cause disease under normal conditions, except when immunity is impaired or the vaginal mucosa is damaged. However, the impact of these infections to female infertility is still unclear. Some authors reported that U. urealyticum and M. genitalium may cause potential pathological effects on fertility in women [5, 8], while other research has indicated no relation [9]. Whether U. urealyticum and M. genitalium are responsible partly for infertility or whether this relationship is only coincidental remains an unanswered question.
Currently, it is not recommended for routine screening of asymptomatic individuals for Mycoplasma hominis, U. urealyticum, and U. parvum, because the asymptomatic presence of these bacteria does not always develop disease. However, cases with a high U. urealyticum load may be benefit from treatment [10]. The widely indication of these testing, diagnosis and accordingly treatment of these bacteria may result in economic burden [10]. However, while detection via PCR at a certain time cannot be causally linked to prior damage, the presence of M. genitalium or U. urealyticum may be associated with prior infection with a traditional sexually transmitted urethritis-inducing agent, such as Neisseria gonorrhoeae or Chlamydia trachomatis, which may be related to secondary infertility. Furthermore, new techniques of molecular methods have made the detection of U. urealyticum and M. genitalium more feasible at high sensitivity and specificity. So far, the recommendation of screening U. urealyticum or M. genitalium to prevent the complication of PID with severe tubal damage is still controversial. The present study aimed to determine the prevalence of U. urealyticum and M. genitalium infections in cervical samples from secondarily infertile women and their relationship to fallopian tube disorders.
2. MATERIALS AND METHOD
Women with secondary infertility who underwent treatment at the Center for Reproductive Endocrinology & Infertility, Hue University Hospital, from July 2017 to July 2018 were enrolment for a cross-sectional descriptive study. Inclusion criteria included women who were unable to get clinical pregnant after 12 months of active sexual intercourse or the inability to get a full-term pregnancy until a live birth. Exclusion criteria included being treated for genital tract infection with local or systematic antibiotics within 4 weeks prior to inclusion, menorrhagia or declining enrollment. The local ethics committee of Hue University of Medicine and Pharmacy has approved this study, registration number H2016/243. All participants were explained and requested for written informed consent.
All recruited women were interviewed following a prepared protocol to obtain general characteristics including age, occupation, geography, history of miscarriage, and history of genital tract infection. A standardized pelvic examination was then performed, and one vaginal swab was taken for direct microscopic examination by wet mount. One endocervical sample was also collected for detection of U. urealyticum and M. genitalium by PCR.
Hysterosalpingograms (HSGs) were indicated and assessed by experienced doctors in all cases to investigate the cavity of the uterus and fallopian tubes. First, a standard image was obtained before the injection of contrast (Ultravist 300 (iopromide), Bayer, Leverkusen, Germany). Four images were taken to assess the uterine cavity, tubal patency, and the appearance of contrast medium in the pelvic cavity.
Total DNA from the vaginal swab samples were performed using iVApDNA Extraction Kit (Viet A Technology Corp., Ho Chi Minh, Vietnam)., following the manufacturer’ instruction. The quality of total DNA were checked using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Conventional PCR was performed using primers specifically targeting 540 bp of the urease gene of U. urealyticum and 281 bp of the adhesin gene of M. genitalium [11-14].
U. urealyticum
forward: 5′-AGAAGACGTTTAGCTAGAGG-3′ forward: 5′- AGTTGATGAAACCTTAACCCCTTGG-3′
M. genitalium
reverse: 5′-ACGACGTCCATAAGCAACT-3′
reverse: 5′- CCGTTGAGGGGTTTTCCATTTTTGC-3′
A total reaction volume reaction 25-μL reaction contained 5 μL DNA template, 0.4 μM each primer, and 12.5 μL 2× GoTaq Green Master Mix (Promega, Madison, WI, USA). PCR conditions for detection of U. urealyticum were as follows: initial denaturation at 95°C for 4 min, 36 cycles of 95°C for 50 s, 55°C for 50 s, and 72°C for 60 s. Those for detection of M. genitalium were as follows: initial denaturation at 95°C for 5 min, followed by 36 cycles of 95°C for 30 s, 65°C for 30 s, and 72°C for 30 s. PCRs were performed on a Veriti™ 96-Well Thermal Cycler (Thermo Fisher Scientific). PCR products were separated by 1% agarose gel electrophoresis containing 1× GelRed™ (Biotium, Fremont, CA, USA) and digitalized with a Gel Doc XR System (Bio-Rad, Hercules, CA, USA).
All analyses were performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). An independent sample t-test was used for normally distributed data or the Mann–Whitney U-test for skewed data in comparison of continuous variables between groups., the chi-square or Fisher’s exact test were used for comparing categorical variables. Results are presented as odds ratios (ORs) with 95% confidence intervals (CIs). p value under 0.05 were considered significant statistically.
3. RESULTS
By screening all women with secondary infertility who were treated at the Center for Reproductive Endocrinology & Infertility, Hue University Hospital, from July 2017 to July 2018, total of 95 cases were satisfied inclusion criteria with mean age of 32.36 ± 4.72, 56.8% work in office, 63.2% lives in non-urban area, 60% has educational levels from college grade and 61.1% is secondary infertility more than three years. There is 23.2% women with history of GTI and 63.2% with history of miscarriage. Table 1 also shows that 73.7% of patients had a normal vaginal microbiota. Among the 25 cases with abnormal wet-mount, the most frequent infection type was bacterial vaginosis (17.9%), with candidiasis accounting for 7.4% of cases, aerobic vaginitis accounting for 2%, and trichomoniasis accounting for 1.1%. Two cases of aerobic vaginitis are co-infected with Candidiasis.
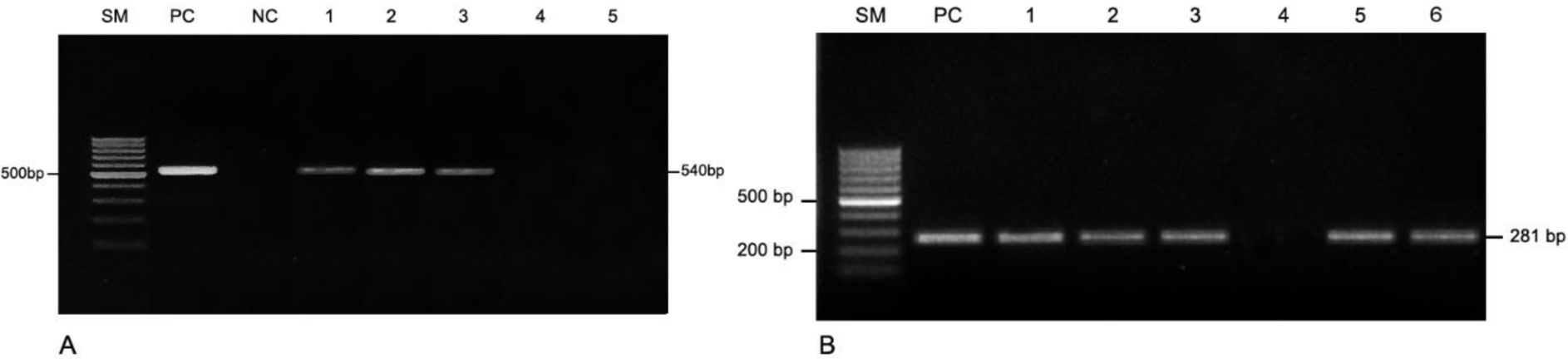
According to PCR of cervical samples from 95 secondarily infertile women, we detected 36 cases (37.9%) positive for U. urealyticum and only two cases (2.1%) positive for M. genitalia (Figure 1). There was no statistically significant association between groups with positive PCR for U. urealyticum as well as group with positive PCR for M. genitalium and age, geography, occupation, education level, history of genital tract infection, history of abdominal surgery, or history of miscarriage, as shown in Table 3. Positive PCR result for U. urealyticum was found to be associated with bacterial vaginosis, with 2.86 times of higher rate of coinfection (95% CI: 0.976–8.367, p = 0.05) while the two cases positive for M. genitalium had normal vaginal microbiota.
Among the 95 cases, normal HSG results were recorded in 64 cases, accounting for 67.4% of patients. Tube occlusion on one or both sides was observed in 13.6% and 9.5% of cases, respectively, and hydrosalpinx on one or both sides was observed in 5.3% and 2.1% of cases, respectively (Table 2). Analysis showed that a positive PCR result for U. urealyticum was correlated with an abnormal HSG in secondarily infertile women, increasing the rate of an abnormal HSG result by 2.88-fold (95% CI: 1.18–6.99, p = 0.018) (Table 4).
4. DISCUSSION
U. urealyticum and M. genitalium have been reported to be associated with female infertility in some studies [15, 16]. M. genitalium has been detected in 5.7% (10/176) of the total population and in 5.6% (9/161) of those with symptoms, corresponding to 5.7% (5/87) of symptomatic men and 5.4% (4/74) of symptomatic women [17]. Ureaplasma was also diagnosed in 25.8% of patients with genital tract infections and 20.8% of infertile women [18]. According to Peerayeh et al., 30.7% of infertile women were positive for Ureaplasma or Mycoplasma, among which 51.7% were positive for Ureaplasma, 26.7% for Mycoplasma, and 21.5% for both [19]. Similarly, Grzesko et al. found a prevalence of M. genitalium infection of 19.6% in infertile women [5]. A population‐based study of M. genitalium in Vietnam reported a significantly low prevalence in reproductive age women in rural areas (0.8%; 95% confidence interval, 0.25–1.35%) [20].
Our study revealed that the prevalences of U. urealyticum and M. genitalium in cervical samples of secondarily infertile patients were 37.9% and 2.1%, respectively. Compared to community studies of infertile individuals, this shows a similar or higher prevalence of U. urealyticum infection. Atefeh et al. reported similar results, with prevalence of U. urealyticum and M. genitalium of 37.5% and 2.9% in 104 infertile women [21]. Sleha et al. found that the prevalence of U. urealyticum infection in 111 infertile women was 39.6% [22], and Melih et al. found that a U. urealyticum infection rate of 42% among women with unexplained infertility [6]. Seifoleslami et al. found that the prevalence of U. urealyticum in infertile women was significantly higher than that in women without infertility [3], and this pattern was also observed in a study by Al-Kayat with 150 infertile and 150 non-infertile women showing prevalences of 22% and 4.7%, respectively [5]. Grześko et al. reported that subjects with vaginal discharge showed a significantly higher incidence of U. urealyticum than the asymptomatic group [7]. A study by Benu et al. in adult women with abnormal discharge revealed that the prevalence of U. urealyticum infection as detected by PCR was 45% [18].
In fact, as demonstrated by the abovementioned studies, research has shown a high prevalence of U. urealyticum in cervical swaps. However, there is less evidence determining the relationship between the presence of a microorganism as detected by PCR of a sample taken at a given time and an acute infection. According to Horner et al., routine testing of asymptomatic persons for U. urealyticum is not recommended. This is because detection by PCR shows the presence of a microorganism but does not confirm an infection and therefore cannot indicate whether the microorganism has caused tubal damage prior to testing for infertility [10].
In our study, among women with a positive PCR result for U. urealyticum, the prevalence of an abnormal HSG result was 47.2%, while in women without a positive U. urealyticum result, the prevalence of an abnormal HSG was only 23.7%. The two patients with M. genitalium infection had normal HSGs. There was a statistically significant relation between U. urealyticum infection and an abnormal HSG. An early study by Henry-Suchet et al. performed laparoscopic examination on 99 women in three groups: (1) 17 with acute pelvic inflammatory disease, (2) 46 with tubal infertility without pelvic inflammatory disease, and (3) a control group of 36 women with infertility due to other causes [23]. The results showed that U. urealyticum was present in 17% of the specimens collected from the fallopian tubes or peritoneum of the experimental groups and only 5% of those from the control group. Among infertile women with occluded fallopian tubes, the prevalence of U. urealyticum infection was five times higher than that of the infertile women with normal tubes, but this difference was not statistically significant [24]. A study in Spain found that in infertile women, Ureaplasma infection rates were 21.7%, and among 10 cases with Ureaplasma present, an abnormal HSG was recorded in seven cases [25]. However, this statistically difference was not significant due to the small sample size.
Studies on M. genitalium have also shown high infection rates in infertile patients. A study by Nonika and colleagues in women with infertility in India showed that the prevalence of M. genitalium infection was 16% among specimens from the urine, cervix, or endometrium, while no infections were detected in non-infertile women [26]. Rates of occlusion and endometrial hyperplasia were 33% and 26.66%, respectively, among those positive for M. genitalium [26]. Svenstrup et al. studied 194 women with tubal disease and showed that 17% of patients had anti-M. genitalium antibodies, compared with only 4% of women with normal uterine tubes; additionally, 14% of patients with a history of pelvic inflammatory disease were positive for M. genitalium, compared with 6% of patients with no history of PID [27]. However, in our study, only 2/95 women with secondary infertility were positive for M. genitalium, so it was not possible to assess associations with other factors.
Limitation of the current study is that the detection of M. genitalium and U. urealyticum by PCR will show the presence of a microorganism but not infection and not ensure that the microorganism has caused tubal damage prior to testing for infertility. Therefore, it would be more relevant to test for antibodies to the microorganisms of interest, as the presence of antibodies would indicate a previous infection. In addition, abnormal HSG findings could also be caused by other co-infections not tested for, such as C. trachomatis and N. gonorrhoeae.
In conclusion, previous research has revealed important complications involving U. urealyticum and M. genitalium infection, but prevalence appears to vary in different populations. Our data showed that in secondarily infertile women, the presence of M. genitalium was rare whereas the proportion positive for U. urealyticum according to PCR was high. The presence of this bacterium may be associated with tubal damage. It is therefore necessary to routinely screen for U. urealyticum for better diagnosis and management of secondary infertility.
