1. INTRODUCTION
Obesity, which is usually classified by the Body Mass Index (BMI), a major health problem in several countries, leading mainly to cardiovascular problems and premature death [1], besides being also one criterion of the metabolic syndrome definition [2].
It is well-known that thyroid hormones influence the differentiation, energy metabolism and growth of almost all cells and tissues. This system is based on central regulation by the hypothalamic–pituitary–thyroid (HPT) axis that maintains serum levels of thyroid hormone at remarkably stable levels through the actions of Thyroid Stimulating Hormone (TSH) and TSH-releasing hormone (TRH) [3].
The HPT axis is negatively controlled by thyroxine (3,5,30,50-tetraiodo-L-thyronine (T4)) and 3,5,30-triodo-L-thyronine (T3) in the circulation. Put differently, T4 mediates TSH secretion suppression, process that also includes the conversion of the first to T3 in pituitary cells; equally, TRH secretion suppression is mostly mediated by this same conversion in hypothalamic TRH neurons. In such equilibrium, an increase in T4 circulating levels and the consequent increase in intracellular concentrations of T3 in hypothalamic TRH neurons and pituitary thyrotropes shut down the TRH and TSHβ encoding genes expression, whereas a drop in T4 circulating levels results in the opposite effect [4].
The hormonal secretion of the thyroid gland is constituted by T4 in 93% and by the nuclear active form, T3, which constitutes 7%. The main pathway of thyroid hormones’ action is at the genomic level by regulating gene expression; and the mechanism that has been explained is through the binding of T3 to the nuclear TH receptor (TRs) which function as a ligand modulated transcription factor [5].
Once inside the cell, the thyroid hormone concentration is tightly controlled by three iodothyronine deiodinase enzymes (DIO1, DIO2 and DIO3) [3]. The first two catalyze the 5’deiodination producing T3 from T4, while DIO3 catalyzes 5-deiodination inactivating T3 and T4 [6], as a result, cells that express D2 have higher T3 levels. The action of deiodinases (DIO2 and DIO3) is extremely important at the brain level to maintain adequate T3 levels [7].
The three DIOs are regulated by thyroid status and many other situations and are present in different tissues. For example, while DIO1 is mainly expressed in kidney, liver and thyroid, DIO2 has been detected in brain, brown adipose tissue (BAT), heart, muscle, pineal gland, pituitary, placenta and thyroid [8].
Many factors are involved in the regulation of deiodinases: biliary acids, cAMP, catecholamines (norepinephrine), insulin and thyroidal status, most of them have been described in several tissues and in BAT or brown adipocytes. Glucocorticoids are also strong regulators of DIO2 activity and mRNA expression in brown adipocytes [6].
According to another text, there could be a variation of thyroid hormones by a DIO2 polymorphisms [9]. What is clear is that, beyond hypothyroidism, one of the causes of obesity could be a thyroid disease [6]. Surprisingly, almost all studies about low T3, explain this laboratory finding as a consequence of an acute health distortion, but the case we report, raises the possibility that it may precede some cases of obesity.
2. CASE REPORT
A 59 years old male patient with obesity since childhood, accentuated when he reached the age of thirteen; for which he Table 1. Thyroid profile has gone to different clinics both in Mexico and in the USA. He developed Type 2 Diabetes Mellitus (T2DM2) at the age of 30 years, treated first with metformin and recently (ten months) with Tresiba (insulin degludec) 30 U per day. Other medications he usually takes: Trental (Pentoxifylline) 400 mg, one and a half tablet every 12 hours, Tenormin (atenolol) 50 mg every 12 hours and Aspirin 100 mg every 24 hours.
On May 2019 he fell down in his house due to apparent hypoxic encephalopathy as consequence of chronic apnea. He spent two days on the floor before being hospitalized but it could not been ruled out brain damage for not having a Computed Tomography equipment that could support his weight (approximately 200 kg).
In the biochemical profile, it was documented HBA1c: 7.1%, creatinine: 1.4 mg/dL, Na: 142 mmol/L, K; 4 mmol/L, Hb: 12.8 g/dL, mean corpuscular volume: 72 fl, total cholesterol: 234 mg/dL, C-reactive protein: 47 mg/L, AST: 506 U/L, ALT: 396 U/L. Immediately, it called our attention the persistent low T3 levels with the rest of the thyroid profile in normal ranges (Table 1).
His weight was confirmed following the next steps: Due to his limited mobility, he was taken by car to a commercial load scale where the car was weighed first with him and then only the car, giving a difference of 180 kg, and a Body Mass Index (BMI) with a height of 1.68 m of 63.77 kg/m2. The levothyroxine dose to start treatment was 25 μg per day. To prescribe an individualized diet, the patient’s personal caregiver (who was living with the patient), registered the food consumption during one month (Figure 1), showing that the total diary amount of kcal was below 2000 kcal, not corresponding with his physical status.
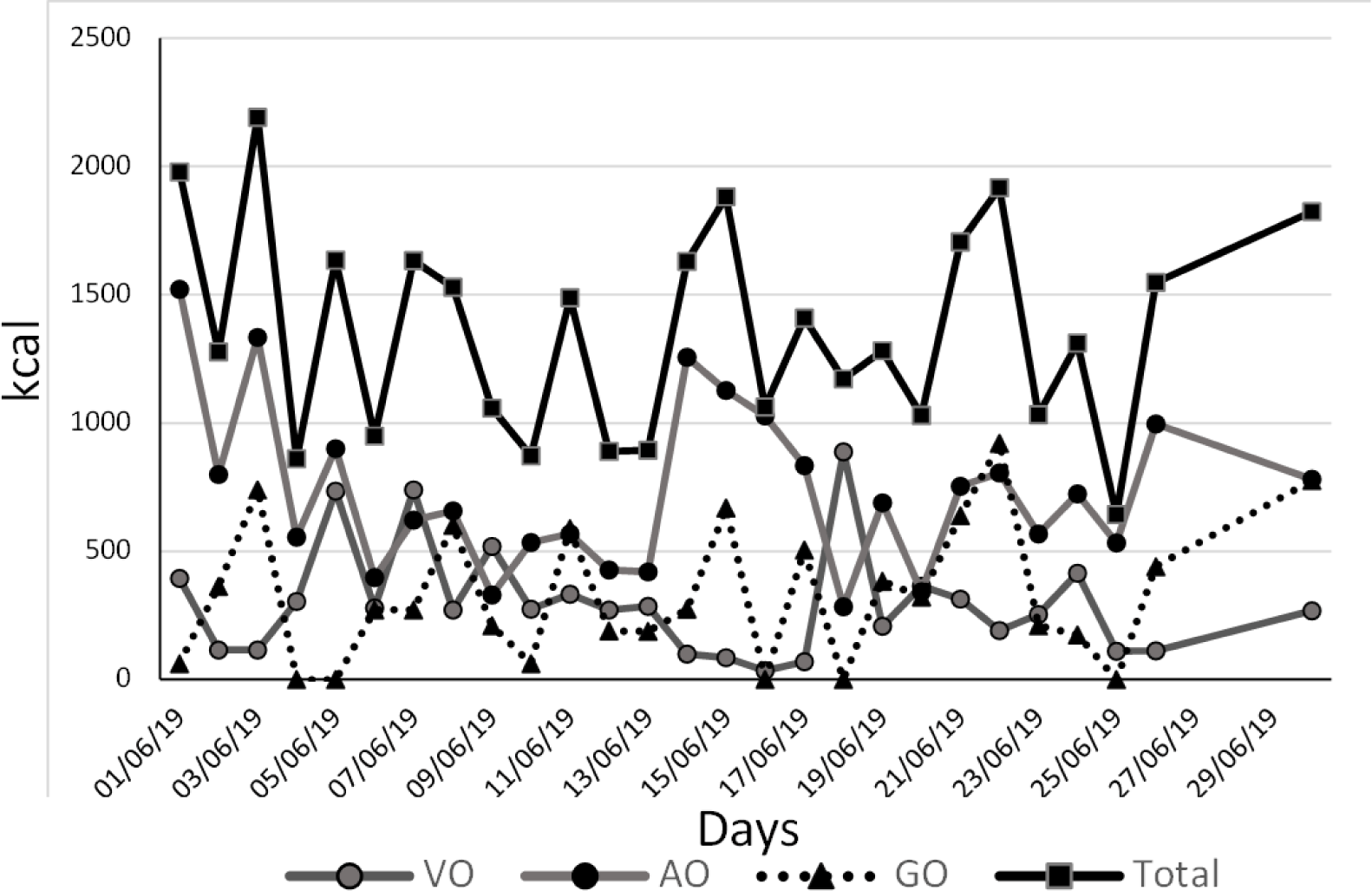
After two months of the hospital discharge the hepatic profile recovered normal values as did the hematic cytometry. Serial thyroid profiles were taken and at six months the T3 was of 6.32 ng/dL (reference range 8-12 ng/dL) in a second Laboratory and at 8 months after the acute episode it was of 46.14 ng/dL (reference range 58-159 ng/dL) in a third Laboratory, reflecting all of them again a low T3 level. Furthermore, the complementary thyroid studies were: DIO2: 475 pg/ml (reference range (143.29-466.21)) and reverse T3: 12 ng/dl (reference range 8-25). Complementing the information, the fT3/T3 ratio went from 4.91 to 8.89 during his hospitalization (May 2019) and in November 2019 it dropped to 0.04. His updated weight at this time was of 190 kg, BMI: 67.31 kg/m2.
3. DISCUSSION
The majority of the cases about morbid obesity can be associated with eating disorders and after a long time the thyroid profile results are affected [10] but in our patient, it seems to be a problem of low T3 since childhood. For this reason, a bibliographic research was performed in Pubmed, COCHRANE Library, Scielo and ScienceDirect for three concepts (obesity, deiodinases, low T3 syndrome), finding that almost all studies talk about the decrease in T3 levels in cases of hospitalization but none as the primary cause of obesity [11-13].
The euthyroid sick syndrome is a common thyroid alteration found in acute illness [14]. The study of Keskek et al. suggests that inflammation, which is strongly associated with adipose tissue, may lead to nonthyroidal illness syndrome (NTIS), including low T3 in obese patients without any comorbid disease [15]. Again, the case of this report illustrates that the low T3 syndrome could be more prevalent than expected in non-hospitalized patients and contribute to the weight increase due to a low metabolic index.
Poor thyroid function contributes to weight gain, and in line with this case report, Ahmadi et al. have published that lower Sex Hormone Binding Globulin, leptin, T3 and UCP2 levels may decrease the Resting Energy Expenditure (REE) level in obese women [16]. In consequence, the REE might be reduced in our patient.
In a review of thyroid dysfunction in obese and overweight children, Witkowska-Sędek et al. stated that the slight hyperthyrotropinaemia and a moderate increase in total T3 and fT3 concentrations are the most common hormonal abnormalities in obese children [17]. This is clearly opposite to what suggested as cause of chronic with our case, which shows chronically decreased T3 levels.
Energy expenditure raises with the fT3/T3 ratio increment showing a positive correlation with basal metabolic rate, total energy expenditure, and sleeping energy expenditure [18, 19]. The low value of this ratio in our patient is consistent with the hypothesis of decrease energy expenditure he has suffered since childhood.
Fasting results in decreased T3 production, and, consequently, significantly decreased serum T3 concentrations [19]. The latter can be applied to our patient when corroborating with low vigilance the low caloric intake that did not correspond to his BMI.
As exposed previously, there are several steps that lead to the correct T3 synthesis and secretion. In this regard, it was explored the possibility of a predominant conversion of T4 into rT3 or a failure in DIO2, both options discarded with the respective measurements. The normal values of rT3 discard also a higher T3 clearance of plasma due to D3.
A limitation of this report is the missing information about many hormones that could alter the thyroid hormone synthesis process such as leptin, but a clear strength is the individualized supervision with a hard strategy of assigning a health assistant to live with the patient proving a lack of correlation between the weight status and the daily total calorie intake of the patient.
4. CONCLUSION
Under certain circumstances such as in this case, chronic low T3 levels might affect energy metabolism and as such BMI or risk for obesity.
We propose the low T3 syndrome as cause of obesity when it is confirmed a total kcal ingestion below the expected for the actual BMI, in the absence of acute or chronic conditions.
