1. INTRODUCTION
Over the past three decades, Vietnam has attempted to impede the HIV spread and its burdens on the people’s health. Effectively action programs were launched on the national scale which resulted in a decrease in the number of new cases and deaths by October 2019 compared to the peak period of the HIV epidemic (approximately 10,000 and 103,426 vs. 30,000 and 15,000 per year, respectively) [1-4]. Injected drug users (IDU) were no longer the majority of the HIV spreading source and the key populations were shifted into female sex-workers and people having male homosexual contacts [4, 5]. Currently, the distribution of new infections had shrunk and found mainly in the north and the south of Vietnam [4-6].
However, Vietnam is facing a great financial challenge which may impede the process to achieve its 90-90-90 goal by 2030. Before 2020, the HIV/AIDS response in Vietnam had relied mainly on external donors, therefore, most preventions and treatments were free of charge. In 2008-2012, about 85% of the cost of the drugs and 70% of the preventions and control programs were financed by the United States President's Emergency Plan for AIDS Relief (PEPFAR), the World Bank, the United Kingdom Department for International Development, the Global Fund, the Asian Development Bank, and many others [7]. In Ho Chi Minh City (HCMC) - the biggest city of Vietnam - international donors accounted for 94.5% of the total funding for HIV/AIDS prevention and control in the period of 2008-2013 [8]. All external funding was withdrawn by 2020 and the HIV/AIDS response had to be covered by the domestic resources and private sector. To help HIV-infected populations access HIV care services at a lower cost than the cost of paying out-of-pocket, the Ministry of Health (MOH) implemented the Plan on Financing the HIV/AIDS Prevention and Control Activities in the period of 2014-2020. In this Plan, the HIV/AIDS care services for people living with HIV (PLHIV) have been integrated with the existing social health insurance (SHI) scheme of the Vietnam Social Security (VSS). The SHI whereby has covered 100% of the treatment cost for special HIV-infected populations such as children under 6 years old, poor populations, and ethnic minorities; 95% for the near-poor and retirees; and 80% for the remaining of the HIV-infected population. In 2020, the MOH’s goal is to have at least 150,000 PLHIV participating in the SHI scheme [4].
Although the integration of PLHIV into the SHI scheme seems to be relevant and feasible in the context of current policy frameworks for SHI and HIV/AIDS control, its impact on the SHI budget is not well-understood. Several studies have been conducted to examine the cost of these programs, however, their approaches were from a social perspective instead of the VSS perspective [9-12]. Additionally, no national evidence is available to identify cost drivers among cost components. A budget impact analysis of the HIV/AIDS treatment on the national scale could aid decision-makers to estimate the financial consequences of integrating PLHIV into the SHI scheme and allocate resources in a short-term horizon (e.g., 1 to 5 years). To support the VSS in making decisions on payment policy for HIV/AIDS patients in the next few years, this study aimed to analyze the budget impact of the treatment for adult patients on the national scale in the past four years (from 2016 to 2019) if the SHI covered all the costs against if the SHI covered nothing. This study also analyzed the budget impact of the treatment for 150,000 adult patients in the year 2020 if the SHI covered all the costs against if the SHI covered nothing, from the VSS payer perspective.
2. MATERIALS AND METHODS
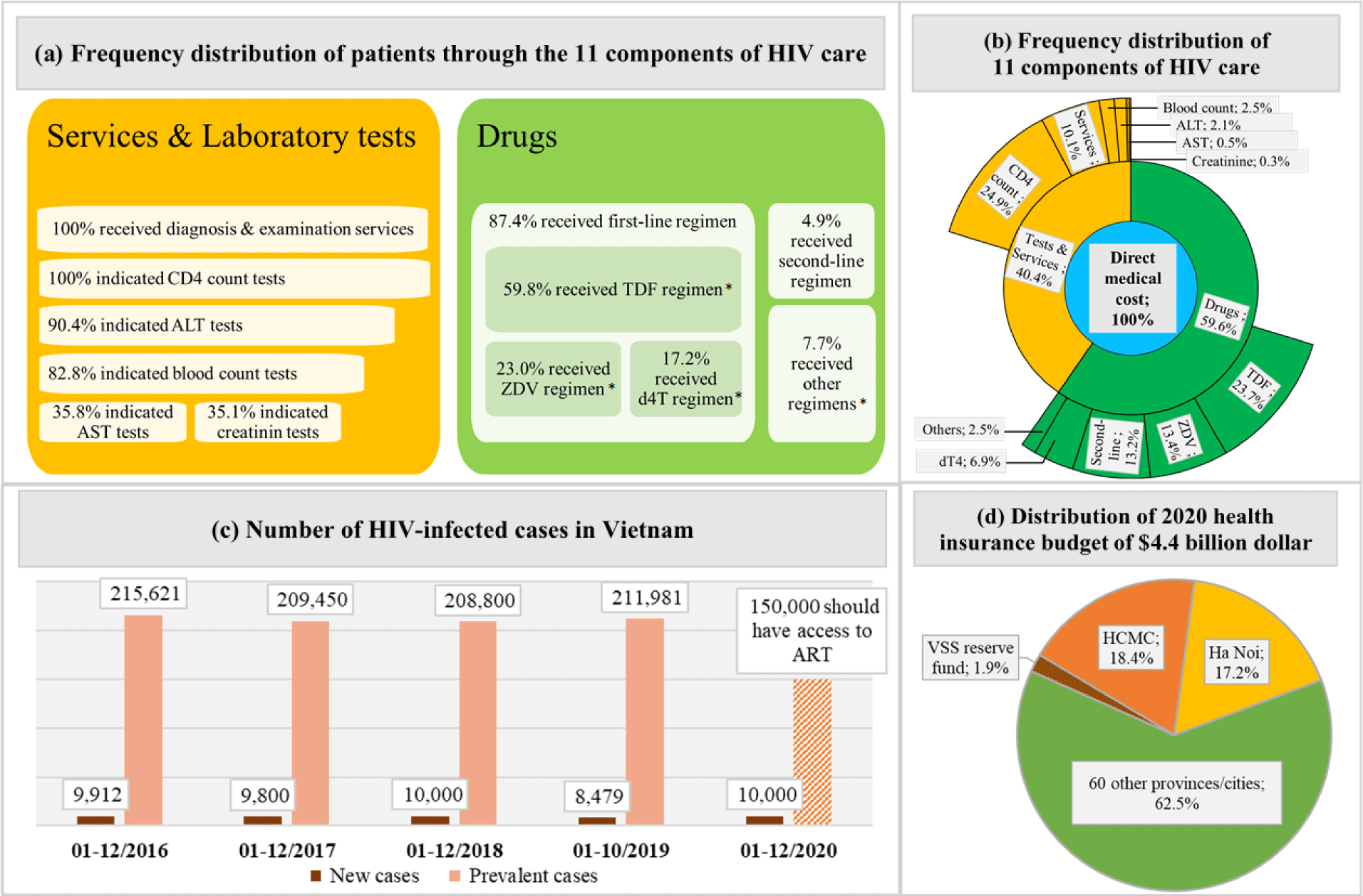
We developed a spreadsheet-based budget impact analysis model with a 5-year time horizon from the VSS perspective. The model was constructed based on two types of data sources. First, a 13-year administration data of the Medical Center of District 1 in Ho Chi Minh City (MCD1HCMC) was used as a cost data source to extract types of treatment cost and their mean values and calculate frequency distributions of types cost and their patients [13]. This database contained all HIV-infected patients aged 18 years or older and received ongoing ART for the first year of treatment at the MCD1HCMC. The MCD1HCMC was one of the health facilities providing HIV care and receiving support from the PEPFAR since 2006 and its data on managing HIV/AIDS patients had been collected and accumulated. A total of eleven cost components directly related to HIV/AIDS treatment was extracted and divided into two cost groups which were drugs cost and services and laboratory tests cost. The total direct medical cost (DMC) was the sum of two cost groups or of eleven cost-components. Second, data from annual healthcare summary reports of the MOH from 2016 to 2018 and the report on results of HIV/AIDS prevention and control in 2019 were used as a census data source to extract the number of prevalent and new HIV-infected cases each year [4, 14-16]. The spending distributions of the SHI budget in this study were based on the Decision number 163/QD-TTg on the assignment of the SHI budget to pay for diagnosis and treatments in 2020. According to this Decision, 4.4 billion dollars was the total amount of the SHI budget for the whole nation, in which, each province or each central city was assigned a certain part of the total SHI budget. HCMC and Ha Noi were assigned the largest parts of the SHI budget, which accounted for 18.4% and 17.2%, respectively [17].
Mean (±SD) values per patient of the first year total DMC, its two cost groups, and its eleven cost components were extracted from the MCD1HCMC database, then were multiplied with the total numbers of prevalent and new cases each year, in order to extrapolate the relevant costs for the entire HIV/AIDS population in Vietnam. Budget impacts were described through the proportions of the DMC, drugs cost, and services and laboratory tests cost over the total SHI budget, HCMC and Ha Noi budget, and the VSS reserve fund.
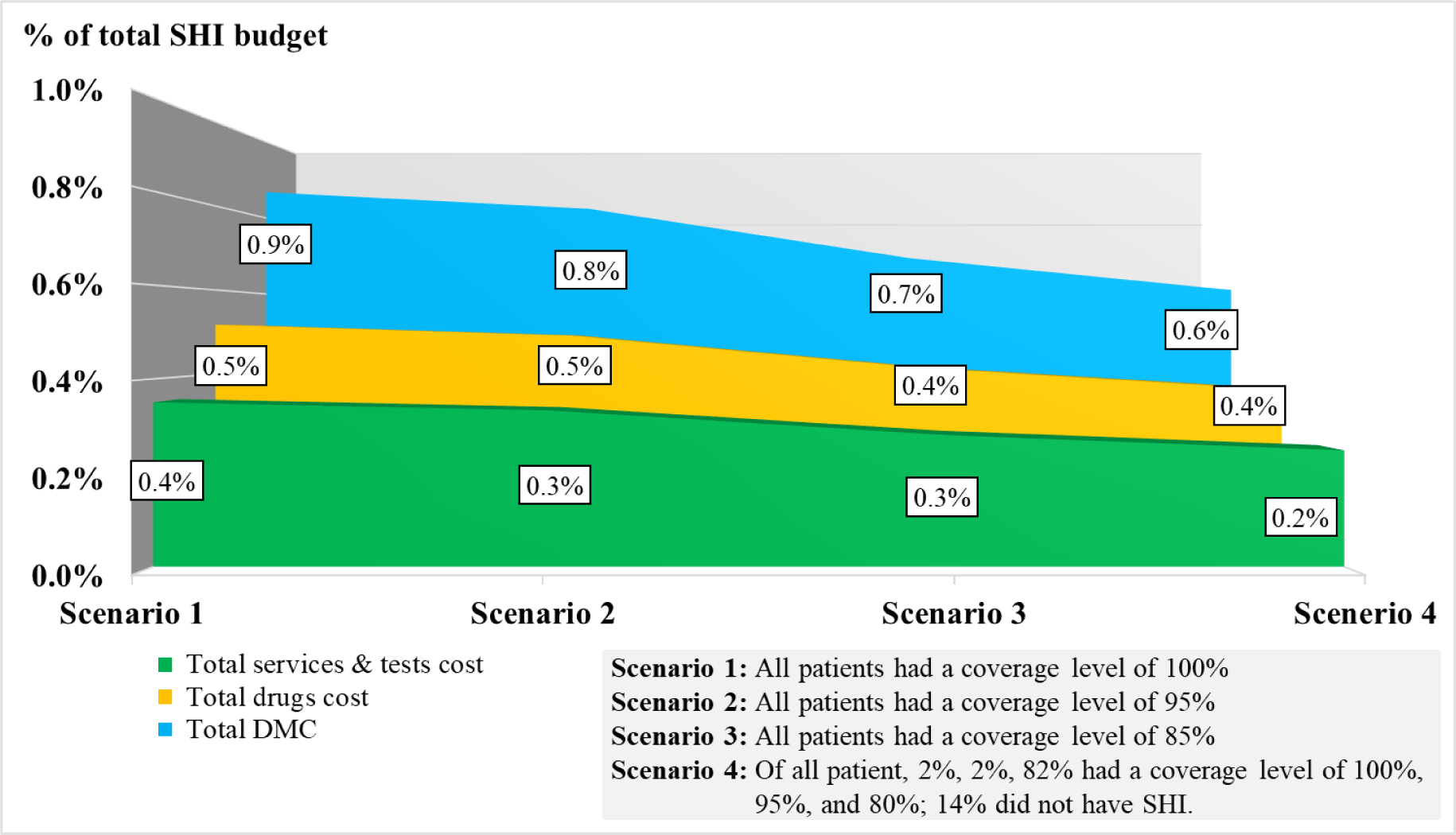
We estimated the DMC, drugs cost, and services and laboratory tests cost for the entire Vietnamese HIV-infected population for the year 2018 in four settings of the scenario analysis. Three first scenarios were based on three different coverage levels of the SHI. The fourth scenario was based on a simulation of the situation at the MDC1HCMC in the period of 2006-2018. Currently, the VSS classifies its SHI members into three coverage levels which are 100%, 95%, and 80% of the total DMC. Of the total HIV-infected patients receiving care at the MCD1HCMC from 2006 to 2018, 2% had an SHI coverage level of 100%, 2% had an SHI coverage level of 95%, 82% had an SHI coverage level of 80%, and 14% did not have SHI.
All costs were calculated according to the unit price of the SHI, then converted to 2020 US dollars. 1-way sensitivity analyses with a Tornado chart were conducted with variations of mean values in a range of ±25%. The statistical analysis was conducted by Microsoft Excel 365.
Figure 1 provided an overview of the data used and the distribution of the national SHI budget in the study.
The research protocol was approved by the Medical Ethics Committee of the MCD1HCMC and the University of Medicine and Pharmacy at HCMC in 2018. The whole research process was conducted confidentially. All the information related to HIV/AIDS treatment at the MCD1HCMC was completely anonymous and served only for research purposes.
3. RESULTS
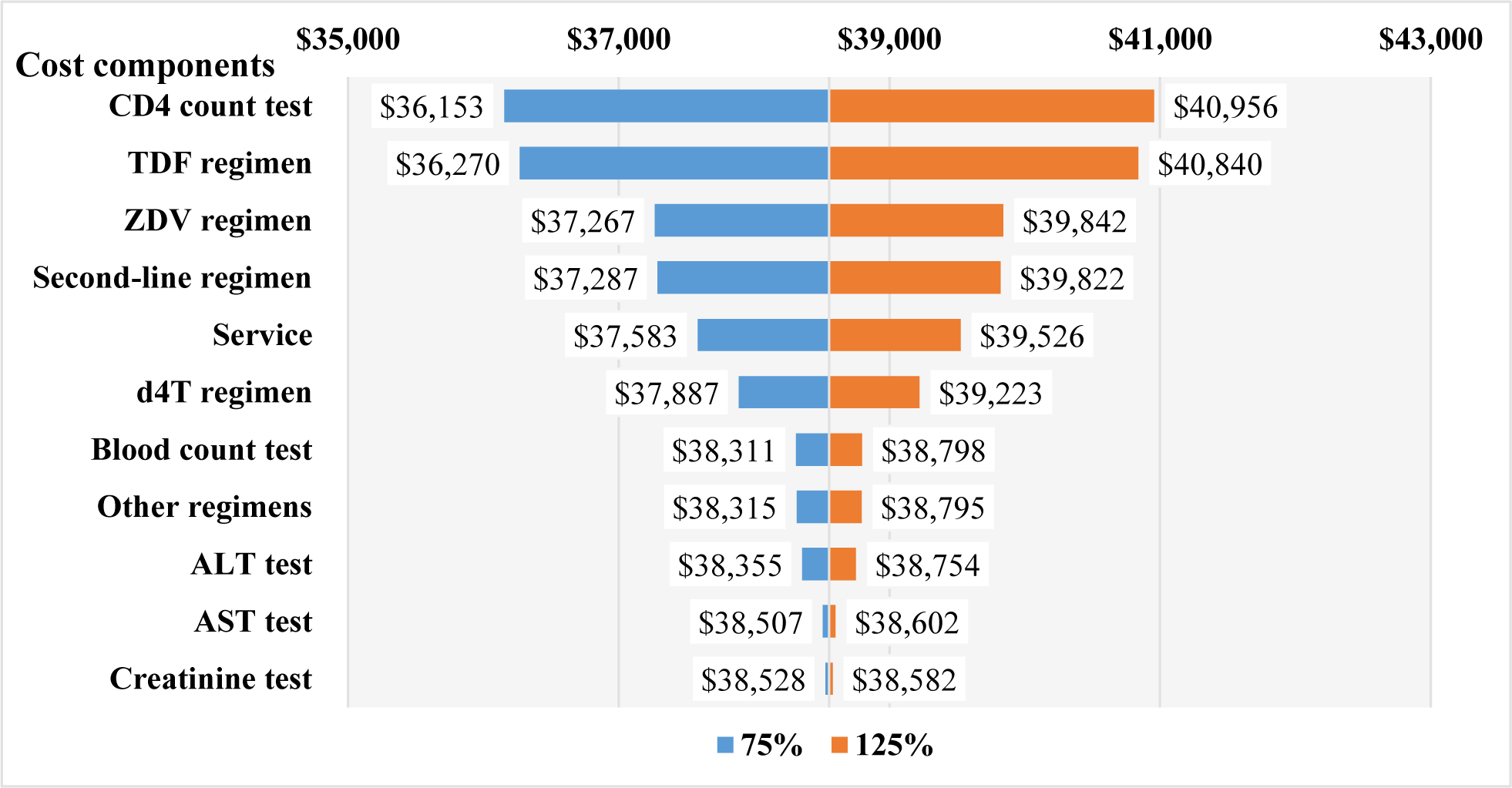
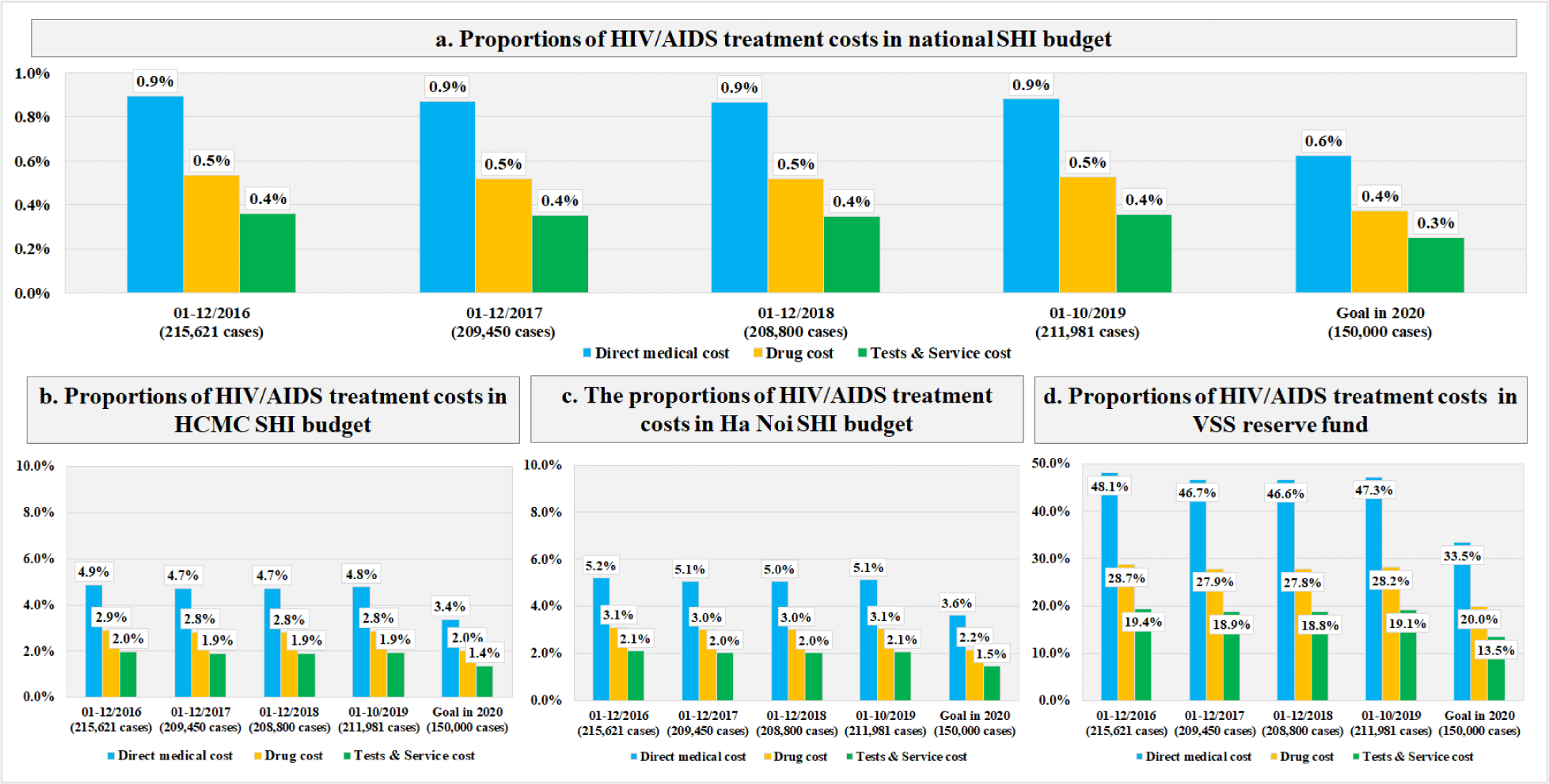
The mean (±SD) value of the first year DMC was $184.6 (±108.6), in which the cost of drugs was larger than the laboratory tests and examination services ($110.1±107.3 vs. $74.5±24.4). For new infections, the total first year DMC did not change significantly, which stayed around $1.8M through the study period (Table 1). For prevalent infections, the first year DMC was $39.8M (215,621 cases) in 2016 and $27.6M (150,000 cases) in 2020 (Table 2). The budget impact analysis showed that, the total DMC accounted for 0.6% of the SHI budget, 3.4% of HCMC, 3.6% of Ha Noi, and 33.5% of the VSS reserve fund to meet the MOH’s goal in 2020 (Figure 2). The costs of CD4-count test and TDF regimen were the major contributors to the total first year DMC each year. Among four scenarios, the budget impact of the DMC of the fourth scenario was the smallest ($27.1M, 0.6%). The drugs cost and services and laboratory tests cost in this scenario were $16.1M (0.4%) and $11.0M (0.2%), respectively (Figure 3). The most influential cost components determined from the sensitivity analysis included the TDF regimen and the CD4 count test, which resulted in changes of ±6.2% and ±5.9% of the total first-year DMC, respectively. (Figure 4)
4. DISCUSSION
This study provided the first-ever evidence base for budget impact analysis of nationwide HIV treatment and care costs in Vietnam from the VSS perspective. Since the costs of HIV treatment per patient in Vietnam were reasonable, the results showed that the impact on the national health insurance budget was small as if all HIV-infected patients getting the treatment free of charge.
The cost inputs of this study were lower than the cost of HIV care reported in published studies, however, we found a similarity in the distribution of cost components. According to a study published in 2003, the medical cost for outpatients at some provinces and cities in Vietnam was approximate $274 during the first year of HIV/AIDS treatment [9]. Compared to studies conducted outside of Vietnam, the cost of ARVs per person per year in low-, middle-, and high-income were $792, $932, and $1,454, respectively, which were much higher than the mean of the total DMC in our study [9]. It cost $682 in South Africa [18], $580 in Indonesia [19], $265 in Tanzania [20], $251 in Kenya [21], and $243 in Zambia [18] during the first six months of treatment. The cost inputs in our study included the cost of laboratory tests, services, and drugs, in which the cost of drugs accounted for 59.6% of the total DMC. The cost of counting CD4 cells was the largest one among other laboratory tests, which accounted for 61.7% of the cost of laboratory tests and services. One study from Uganda showed a similar distribution with the proportion of the ARVs cost, labor cost, laboratory tests, and other costs accounted for 68%, 12%, 10%, and 10%, respectively [22]. Another study from Kenya also revealed that the cost of ARVs accounted for the largest proportion of the total medical cost, which was 50%, 44%, and 49% at each facility [21]. Literature showed the delivery costs for antiretroviral treatment and prevention of mother-to-child-transmission of HIV accounted for the largest proportion of the total direct medical cost, which was 64% among low-income countries, 50% among middle-income countries, and 47% among high-income countries [23]. At the MCD1HCMC, from 2006 to 2018, there were two most common regimens used which were Tenofovir Disoproxil Fumarate+Lamivudine+Efavirenz (TDF+3TC+EFV) (47%) and Stavudine+Lamivudine+Efavirenz (d4T+3TC+EFV) (16%). Our study noted a change in the trend of using first line ARV regimens in the period 2006-2018 with an increase in the use of TDF+3TC+EFV and a decrease in the use of d4T+3TC+EFV. Due to the long-term side effects of dT4 such as lactic acidosis, lipodystrophy, and peripheral neuropathy, since 2011, the MOH had no longer introduced the regimen d4T as the preferred regimen used in HIV/AIDS treatment [24]. Since 2017, the first line ARV regimens recommended by the MOH have been Tenofovir Disoproxil Fumarate/Zidovudine+Lamivudine+Efavirenx/Nevirapine (TDF/ZDV+3TC+EFV/NVP) [25].
After all international funding withdrawal, it is necessary for HIV/AIDS patients to have a sustainable financial resource and continue their treatment or encourage incident patients to seek screening and treatment. Our study was able to confirm the high feasibility and sustainability of the integration of HIV/AIDS treatment and the existing SHI scheme. The treatment cost impact on the SHI budget was reasonable even as if all PLHIV received treatment free of charge. With the total DMC for all PLHIV every year less than 1% of the total SHI budget, the scenario analysis also showed that there was only a 0.3% impact difference between the scenario of 86% of PLHIV having full or partial coverage and the scenario of 100% of PLHIV having full coverage in 2018. In fact, the MOH has put effort to cooperate with the VSS to support the access to SHI for HIV/AIDS patients and conducted centralized procurements of ARV drugs to reduce the ARV drug prices since 2018. As a result, the proportion of HIV/AIDS patients attending SHI has increased year by year and reached 100% in many provinces and cities [25].
Since cost data did not follow the normal distribution, using mean values as cost inputs resulted in uncertainty. Results from the MCD1HCMC showed that mean and median values of cost types varied significantly and the most common percentage of the variation was around 25% [13]. Therefore, this study performed a one-way sensitive analysis with a 25% variation to vary each uncertain cost. The sensitive analysis showed the CD4 count test was one of the cost drivers during the first year of treatment. It was reasonable due to its importance in assessing the response to the treatment multiple times. The frequency of using this test would be less in the following years, therefore, its cost would be less compared to the first year of treatment.
We well-acknowledged that there were many limitations in our study. First, the cost inputs were collected from only one health facility which received the funding from only one external organization. Second, some program costs were not included, for example, costs related to training of health care providers and technical assistance, which were not covered by the SHI yet. Third, our study design did not allow estimation of the costs of special populations (IDU, sex workers, homosexual men, or children) due to a lack of data. Our study design also did not allow to estimate treatment costs of PLHIV covered by SHI each year from 2016 to 2019 in order to keep the computing framework as simple as possible, since the SHI only covered some parts of the HIV/AIDS treatment cost for some types of patients after 2018. Fourth, our study parameters gained from the MCD1HCMC database were used to extrapolate cost values and frequency distributions for the entire country. However, characteristics of the patient sample in this 13-year database were comparable to main characteristics of the adult HIV-infected population in Vietnam [13]. As a result, it was reliable to epitomize the cost of treatment for the adult HIV-infected population. Fourth, we only calculated the cost during the first year of treatment for the budget impact analysis each year, however, we assumed with evidence that the cost in the following years would be less than the first year [9]. Therefore, in this study, we assumed that the mean values of the first year DMC were similar or not significantly higher than those of later years, consequently, we consider the findings of the study robust.
Despite the limitations, our study provided the first-ever evidence base of the budget impact of delivering HIV treatment to adult patients on the national scale. Although the study confirmed the small impact on the SHI budget, it also suggested some potential to further reduce the financial burden; for example, optimize the competitive procurement of ARV drugs, integrating HIV treatment and care into different settings of the SHI, and encourage PLHIV attending health insurance plans. Next studies could further investigate the budget impact on the SHI on key populations or to investigate changes in impact between the first year and subsequent years of treatment.
Conclusion
The study analyzed the budget impact of the HIV/AIDS treatment on the national scale from the VSS perspective. The results showed that the cost of HIV/AIDS treatment was economical and the impact on the SHI budget was reasonable. Findings could be used to advise the MOH in allocating domestic resources and optimizing current programs to establish efficient and sustainable service delivery.