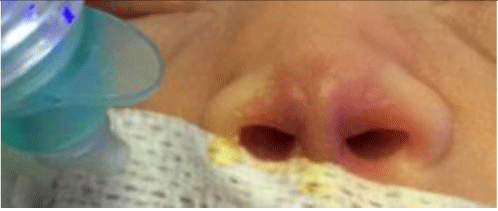
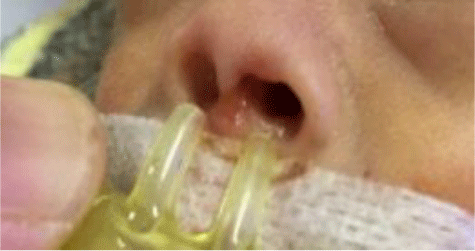
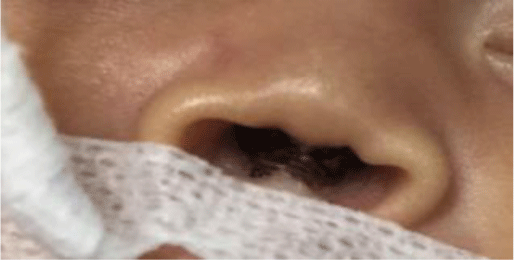
1. INTRODUCTION
Treatment and care for preterm infants have always been a challenge in neonatal intensive care because of the high risks of complication and death [1]. According to WHO, in 2018, there were 30 million preterm infants with low birth weight and other conditions who needed special care for survival [2]. In 2017, approximately 2.5 million neonates died within 28 days after birth [3], of whom 80% had low birth weight and two-thirds were prematurely born. At the Children’s Hospital 1 Ho Chi Minh City, the Department of Neonatal Intensive Care (NICU) admits 1000 to 1200 neonatal patients every year, of whom more than 50% are preterm, low-birth-weight infants. All preterm and low-birth-weight infants upon admittance will receive assisted ventilation at different levels. We usually performed non-invasive ventilation (NIV) as the first-line ventilation-assisting method to minimize ventilation-associated complications. However, despite NIV’s success, we frequently encountered complications caused by NIV interfaces such as nasal ulcers and deformities. Nasal complications may lead to increases in treatment costs, longer hospital stays, and, later on in the infants’ life, psychological trauma. Because of the infants’ deformed noses, it can induce serious worry and concern to the infants and their parents.
At NICU, Children’s Hospital 1, by retrospectively examining health records from 1 April to 15 October 2019, we noted 42 infants had been born earlier than 37 weeks of gestational age. Among them, ten infants had injuries to their nose under nasal cannula NIV, which is a relatively high rate of complications.
Nasal ulcers in infants have various causes: excessively hard cannulae, cannulae too firmly attached which exerts pressure onto the nose’s skin, inappropriate cannula prongs, lack of care instructions regarding prevention of nasal ulcers, lack of frequent examination for skin conditions, and lack of effective nasal dressing pads [4, 4]. In current practice in Children’s Hospital 1, patients usually were given 1-mm thin foam nasal pads that are water-impermeable, rough, and not suitable for protecting nose skin in preterm infants. However, previous studies showed that hydrocolloid nasal pads for preterm infants under cannula non-invasive ventilation could effectively prevent nasal ulcers [6-8]. The hydrocolloid nasal pads are made from a water-resistant hydrocolloid bandage, so it does not easily detach when it comes into contact with nasal secretion fluids. Its outer layer is absorbent polyurethane, hence can reduce pressure-induced trauma on the skin surface. The hydrocolloid pads also had a gel layer that can absorb secreted fluids and maintain skin moisture; it facilitates wound healing and reduces infection. The hydrocolloid pads are safely and firmly attached to the skin surface without skin irritation and does not cause trauma when removed [9].
This study aims to examine the effectiveness of hydrocolloid nasal pads in preterm infants under nasal cannula non-invasive ventilation at NICU, the Children’s Hospital 1 Ho Chi Minh City, Vietnam.
2. MATERIALS AND METHOD
Prospective cohort study with a historical control group. We tracked all preterm infants who were hospitalized at Children’s Hospital 1 Department of Intensive Neonatal Care from 1 November 2019 until 30 April 2020 and underwent NIV with the hydrocolloid nasal pads in use. These patients were monitored for nasal ulceration throughout nasal cannula NIV usage. The prevalence and level of nasal ulceration in the prospective cohort group were compared to that of the control group, consisting of 42 preterm infants undergoing NIV but using thin foam pads from 1 April to 15 October 2019. According to WHO’s definition of preterm birth [12], we subdivided participants based on gestational age: extremely preterm (<28 weeks), very preterm (28–<32 weeks); moderate or late preterm (32–<37 completed weeks of gestation); and on the basis of birth weight: <1000 grams, 1000-1500 grams, 1500-2000 grams, ≥ 2000 grams. Finally, we compared the ratio of gestational age groups by 2 methods.
| Weight of infants (grams) | Size of nasal dressing (width × length) |
|---|---|
| Below 1000 | 1.0 cm × 1.6 cm |
| 1000 to 2000 | 1.0 cm × 2.0 cm |
| Above 2000 | 1.2 cm × 2.2 cm |
Written informed consent was obtained from all participants of the study. A legal representative was explained the benefits, purposes, and disadvantages of participating in the study. Thereafter the representative signed in a written informed consent or had a waiver of the study. This study was approved by the Institutional Review Board of the Children’s Hospital 1 (138/GCN-BVND1, CS/N1/20/37).
Prospective cohort group: All preterm infants (gestational age earlier than 37 weeks), using nasal cannula NIV and hydrocolloid nasal dressings, admitted to the Department of NICU from 1 November 2019 until 30 April 2020.
Historical control group: 42 preterm infants (gestational age earlier than 37 weeks), using nasal cannula NIV and thin foam nasal dressings, admitted to NICU from 1 April 2019 to 15 October 2019.
Historical control: we retrospectively examined 42 cases of infants using nasal cannula NIV in the Department of Neonatal Intensive Care, the Children’s Hospital 1, from 1 April to 15 October 2019. We collected data about their gestational age, weight, nasal skin conditions, and type of cannula used.
Prospective cohort: for all infants who satisfied all our inclusion criteria and no exclusion criteria, we would hand in consent forms to their parents. Afterward, their demographic characteristics were assessed as well as their obstetrical history, gestational age, weight, nasal skin conditions, and type of cannula used. These patients were also monitored for nasal complications (i.e. ulceration) according to the protocols of NICU; this process continued until cannula NIV treatment was stopped or upon the infant’s death. Trained researchers would assess the infant’s nasal skin conditions once a day at a convenient time.
Sample characteristics: The above two groups were similar in the care environment, regimen, and equipment as well as cannulas used in NIV. Indications for NIV were similarly employed, and NICU did not make any change in equipment and care protocols.
When cannula NIV is indicated, nurses will prepare equipment to perform this procedure. Next, nurses assess the infant’s nose size to choose suitable dressings (via weight or direct measurement). Then, nurses wipe dry the infant’s nose. Finally, Peel off the protective strips and stick the nasal dressing onto the infant’s nose. And nasal dressings should conform to the following standards: Suitable size, intact, having unaltered physical properties (i.e., flexible enough), full covering the nose area that comes into contact with the cannula interface (Fig.1).
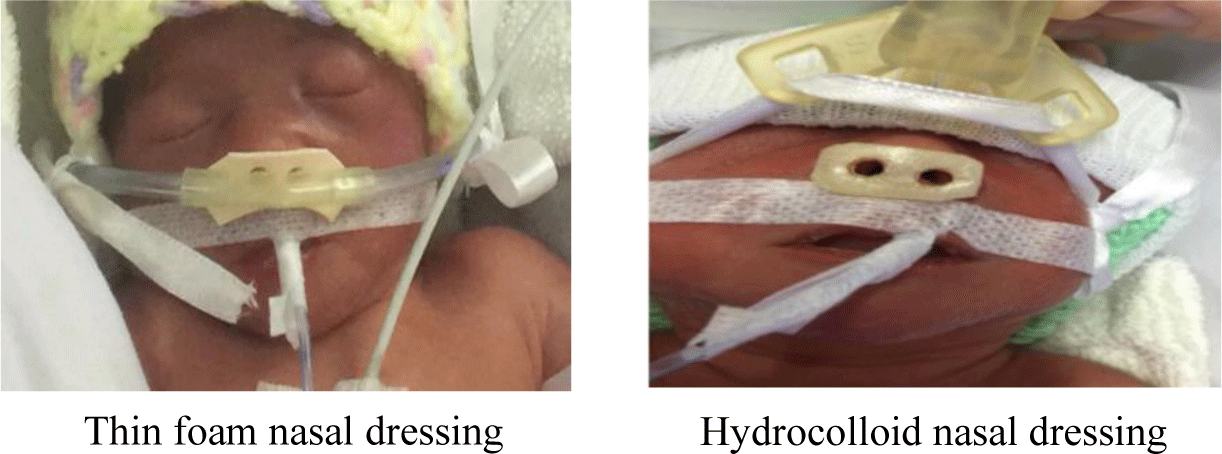
According to the nasal care protocol implemented in NICU, nurses monitor the condition of the nasal dressings every 4 hours, change the dressings every 48 to 72 hours, or when they no longer meet the standards. The infant’s nasal skin conditions were assessed every 4 hours in terms of ulceration severity according to nasal trauma staging (Table 2) so that nasal ulceration might be detected early. The nasal skin conditions serve as a warning sign to nurses to pay more attention to the patients and to inform the attending physician. If necessary, ventilation assisting methods might be changed so as not to cause severe sequelae.
Collected data were checked for completeness and errors after input using Excel. Data analyses were performed using Stata version 13 and R version 3.5.1 following a predetermined plan.
Descriptive statistics: non-continuous variables were presented as frequencies (proportions) corresponding to the two sample groups.
Inferential statistics: Logistic regression models were built to find the relationship between clinical variables and nose ulceration. Univariate analysis was done to explore the association between background variables such as gestational age and the usage of a hydrocolloid or thin foam nasal dressings in relation with nasal ulcers. Subsequently, multivariate analysis was carried out to adjust for the effect of gestational age on the association between the use of hydrocolloid or thin foam dressings and nasal ulcers. The significance level was set at p < 0.05.
3. RESULTS
We recruited 71 infants in the hydrocolloid dressing pads group and 42 infants in the thin foam nasal dressings group. The characteristics of both groups are shown in Table 3.
There were a total of 113 infants participating in this study. In the hydrocolloid dressing pads group, male sex accounted for a higher proportion than the thin foam nasal dressings group (60.5% vs 45.2%), but did not reach statistical significance (p=0.12) . The two groups of participants (with and without hydrocolloid dressings) have similar gestational age characteristics (median: 29 vs. 28 weeks). Birth weights were also similar with the median being 1200 grams in both groups. Besides, in both groups, children with respiratory distress syndrome accounted for a large proportion (69% and 83.3%), and the remaining percentage belonged to other comorbidities, in which pneumonia accounted for 7.1 % in the group with hydrocolloid pads and 4.8% in the group with thin foam pads. Table 3 show that The difference between the 2 groups in participant characteristics when participating in the study was not statistically significant (p > 0.05).
Table 4 shows the prevalence of nasal ulcers by ulcer severity in the hydrocolloid vs. thin foam pads groups. We detected no infant who developed severe ulceration in the hydrocolloid pads group. Among 71 infants using hydrocolloid pads, nasal ulceration was detected in two infants (2.8%), one of whom was mild (28 weeks, 1250 grams), and the other was moderate (26 weeks, 600 grams). In comparison, among 42 infants using thin foam dressings, 10 developed ulcers (23.8%), of whom seven were classified as mild, two moderate, and one severe. However, there was a statistically significant decrease in the severity of nasal injury in infants who had nasal injury in the hydrocolloid pad group compared with thin foam pad group: P = 0.001.
| Ulcer severity | With hydrocolloid pad (n=71) | With thin foam pad (n=42) | p value |
|---|---|---|---|
| n (%) | n (%) | ||
| Mild | 01 (1.4) | 07 (16.67) | 0.001* |
| Moderate | 01 (1.4) | 02 (4.76) | |
| Severe | 0 (0) | 01 (2.38) | |
| Total | 02 (2.8) | 10 (23.8) |
Table 5 presents the prevalence of nose ulcers sorted by gestational age and weight in the two groups. The prevalence of nasal ulcer in age less than 28 weeks was lower in the hydrocolloid pad group compared to that of the thin foam pad group (i.e., 5% vs. 35.7%). Similarly, the prevalence of nasal ulcers in the age of 28 to under 32 weeks was ten times lower in the hydrocolloid pad group compared to that of the thin foam pad group. No patients who were born at 32 to 37 weeks of gestational age developed ulcers in the hydrocolloid pad group, whereas the prevalence was 11% in the control group.
For the weight category, the prevalence of nasal ulcers in “<1000 grams” and “1000 to under 1500 grams” were 4.8% and 3.6%, respectively in the hydrocolloid pad group. In comparison, the prevalence of nasal ulcers in “<1000 grams” and “1000 to under 1500 grams” were 36.4% and 22.7%, respectively in the thin foam pad group.
For both groups, ulcers developed mostly in infants with gestational age under 32 weeks (100% in the hydrocolloid pad group, and 90% in the thin foam pad group). Similarly, ulcers developed mostly in infants with the weight under 1500 grams (100% in the hydrocolloid pad group, and 90% in the thin foam pad group).
Table 4 show that the difference between the 2 groups in gestational age and weight was not statistically significant (p > 0.05).
Table 6 shows the association between gestational age, gender, type of dressing (i.e., hydrocolloid or thin foam pads), and ulcer in preterm infants. Usage of hydrocolloid dressing pads was independently associated with nasal ulceration in preterm infants. The odds of being nasal ulceration in the thin foam dressing pads group was 1/0.09=11 times compared to that of the hydrocolloid dressing pads group.
4. DISCUSSION
Among the 71 participants using hydrocolloid nasal dressings, 2 (2.8%) had nasal ulcers, both of whom had been born before 32 weeks of gestational age (one infant at 26 weeks and the other at 28 weeks) and had lower than 1500 grams of birth weight (one infant at 600 grams and the other 1250 grams). It was found that infants born earlier or with lower birth weight were more likely to develop nose ulcers. This is by the observations in the non-hydrocolloid group: the lower the gestational age and birth weight, the more prevalent nose ulcers became, especially for infants under 32 weeks of gestational age or under 1500 grams of birth weight (Table 5). Additionally, our results indicated a marginal significant reverse relationship between gestational age and nasal ulceration with an OR of 0.81 (0.63, 1.00) (Table 6). Our results were also by those of Celine Fisher et al [8] between 2002 to 2007 in Switzerland. According to the Celine study, which was conducted on 989 infants with an average gestational age of 34 weeks and average birth weight of 2142 grams, nasal trauma was reported in 420 patients (accounted for 42.5%). The risk of trauma to the nose was higher in infants born at less than 32 weeks of gestational age (OR = 2.48) and whose birth weight was less than 1500 grams (OR = 2.28).
Our results showed that the prevalence of ulcers in the hydrocolloid dressings group (2.8%) was lower than in the thin foam dressings group (23.8%) (Table 4). Multivariable logistic regression analysis showed that hydrocolloid pads reduced the odds of nasal ulcer in comparison to thin foam pads, and the reduction was statistically significant with OR = 0.09 (0.02, 0.45) (Table 6). According to a study conducted by Imbulana D.I., Owen L.S. et al. in 2018 [11], out of 108 pediatric patients that were enrolled, 53 used hydrocolloid dressing pads and 55 did not; the rate of ulcer formation in the former group was lower compared to the later group: 18 out of 53 (34%) versus 31 out of 55 (56%).
Severe ulceration can result in the infant losing nasal cartilages, either partly or wholly. This incomplete outer nasal structure affects the respirational function of the outer nose. In addition, when the infant grows up, the aesthetic appearance of the nose is affected, which adversely affects the mental health of the child and their parents. During the research period, our study did not find any severe ulceration when using hydrocolloid dressing pads. This result agrees with the study of Imbulana, D.I, Owen L.S. et al. in 2018 [11].
There are some limitations to our study. First, our study was unable to assess factors that promote the formation of ulcers, e.g. the reuse of nasal cannulas, which makes them lose flexibility and elasticity. Second, there were no standardized, detailed nasal care instructions for infants undergoing nasal cannula non-invasive ventilation (necessary factors include: temporary relief of pressure on the infant’s nose and restoration of blood circulation, nasal hygiene, correct fixation of nasal cannulae and dressing pads, and frequent examination of nose skin). Therefore, there was no better tool for caregivers to give care to paediatric patients. Moreover, the study design was not consistent, and the sample size was small compared to other studies in the world.
Conclusion
Using hydrocolloid dressing pads significantly reduced the prevalence of ulcers as opposed to thin foam pads in preterm infants undergoing nasal cannula non-invasive ventilation, especially for high-risk patients (gestational age lower than 32 weeks, birth weight lower than 1500 grams). More research is needed to evaluate other factors that facilitate nasal ulceration, e.g. the reuse of nasal cannulae, as well as to establish a nasal care protocol for infants using nasal cannula NIV. Clinicians should consider in local hospital, and rate of CPAP-related nasal injury alongside the cost of hydrocolloid dressing when deciding whether to apply this intervention in preterm infants.