1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which caused coronavirus disease of 2019 (COVID-19), was first identified in China on late 2019 [1]. The virus then quickly spread into a global pandemic. Children are also subject to infection with COVID-19. The delta variant of SARS-CoV-2 caused the fourth wave of epidemics in Vietnam with 687.063 cases and 17.090 deaths up to September 19th, 2021. There are about 15.000 infected children in Ho Chi Minh City and the mortality rate of 0.1%. Children infected with SARS-Cov2 are mostly asymptomatic or mild. However, in some cases, children may develop a multisystem inflammatory syndrome that presents clinically as Kawasaki syndrome (KD) [2].
Unlike typical KD group, MIS-C mainly affects children of an older age group [3-7]. Symptoms of KD are observed in many patients with MIS-C; however, gastrointestinal symptoms, such as anorexia, nausea, abdominal pain, and diarrhea were more common in MIS-C than in KD, as shown in 100% of cases in the French study [3-6]. In cases of MIS-C with symptoms similar to those in KD, treatment and follow-up should be quite similar to those in known KD guidelines, such as intravenous immunoglobulin (IVIG) 2 g/kg and low dose aspirin 3-5mg/kg/day [8]. The current medical literature shows that IVIG and methylprednisolone are the mainstays of treatment for MIS-C [8-10]. However IVIG is not always available, especially in low-middle income countries like Vietnam and in remote and isolated areas. Therefore, treatment with methylprednisolone alone in the presence of heart damage is a real challenge.
We report a case of MIS-C with cardiac damage following SARS-CoV-2 infection at Thu Duc City Hospital. Initially, septic shock was diagnosed because it was one of the first MIS-C cases in Vietnam. This case was successfully treated with methylprednisolone alone.
2. CASE REPORT
A six-year-old boy was admitted at Thu Duc City Hospital on August 27th, 2021 for abdominal pain and fever. Three days before admission to the hospital, he had abdominal pain around the navel, intermittent dull ache, not spreading, no pain relief, no vomiting, no bowel movements, sustained fever of 38 - 39°C for 2 days. The abdominal pain was gradually increased with anorexia, so he was hospitalized. He was completely healthy and had never been hospitalized before.
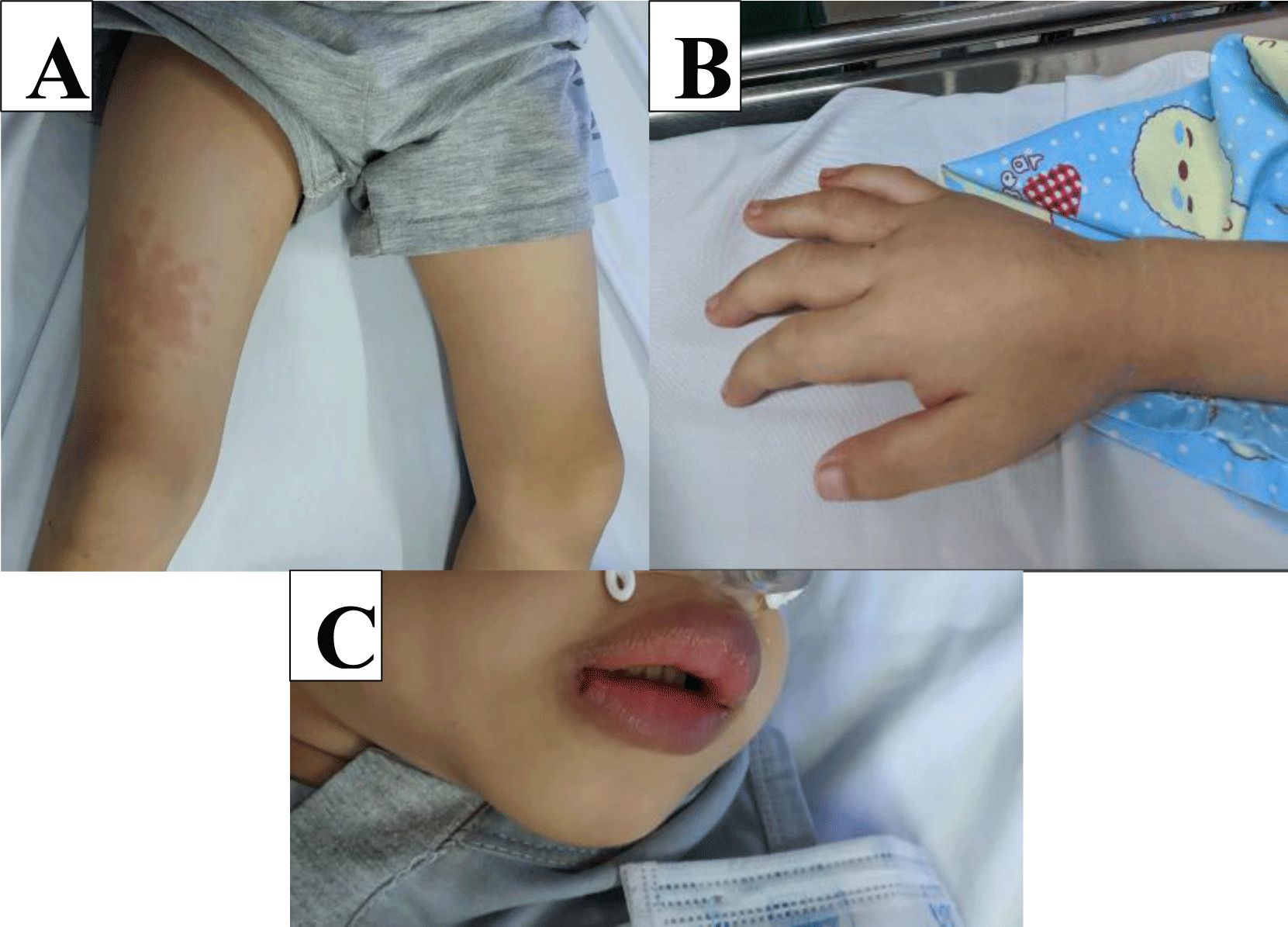
At the time of admission, he had a continuously high fever of 39°C, swollen tonsils, red throat, intermittent abdominal pain around the navel, loose yellow stools, twice a day without blood and mucus, mild eyelid edema, lip swelling, dorsal edema of the hands and feet, erythema 4x5 cm on the front and back of the right thigh, scattered in the abdomen (Table 1, Figure 1). Physical examination noted the symptoms as shown in the Table 2. Laboratory tests showed a hyperinflammatory condition (Table 3). Coronavirus real-time PCR was negative, Dengue NS1 quick test and Dengue IgM-IgG ELISA test were also negative too. Abdominal ultrasound showed lymphadenitis right hip mesentery. The patient was preliminarily diagnosed with sepsis accompanied by acute tonsillitis and treated with antipyretic, antibiotics included: ceftazidime 150 mg/kg/day, amikacin 15 mg/kg/day.
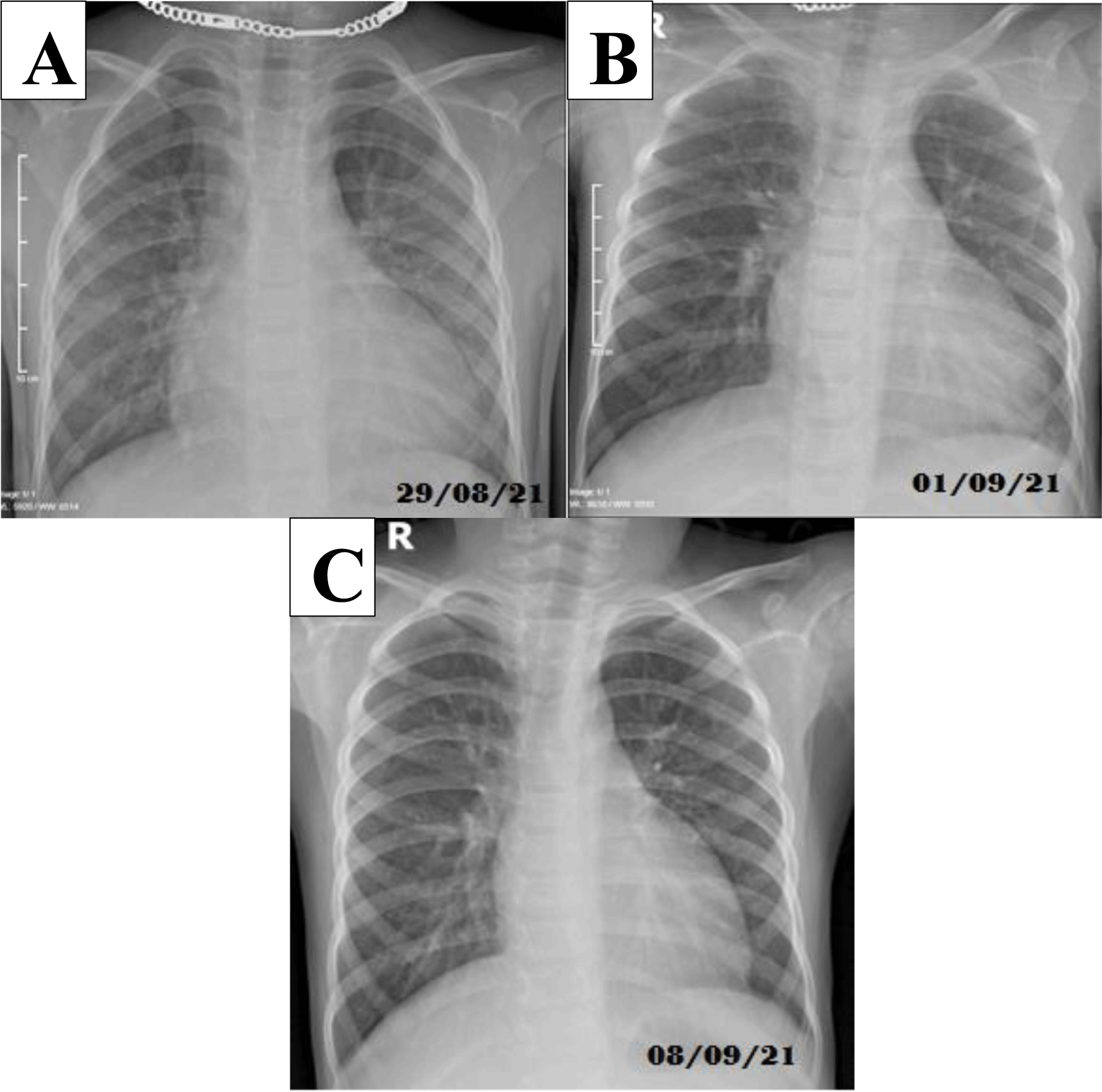
Two days after admission, the patient still had fever, abdominal pain around the navel increased, the liver was enlarged 2 cm below the right lower quadrant, red swollen lips, eyelid edema. Then he had vital signs of shock with blood pressure of 76/50 mmHg, pulse rate of 140 per minute, extremities warm, CRT 2s, respiratory rate of 46 per minute and intercostal contractions. Laboratory test showed a strongly inflammatory response (CRP 325.24 mg/l, procalcitonin 73.25 ng/ml, ferritin 495.7 ng/ml); metabolic acidosis with increased anion gap, elevated lactate (18.9 mg/dL), elevated NT pro BNP (35000 pg/ml) and troponin Ths (54.07 pg/ml) (Table 3). Abdominal CT scan showed hepatomegaly 12 cm in diameter, small amount of peritoneal effusion, no appendicitis; chest X-ray had recorded opacity lesion on both sides of the lung (Figure 2). The patient was diagnosed with septic shock - severe pneumonia that blood culture was negative and treated with oxygen therapy via cannula followed by NCPAP, normal saline infusion 50 ml/kg/1h45, vasopressors include noradrenaline 0.3 g/kg/min, adrenaline 0.3 μg/kg/min, dobutamine 10 μg/ kg/min; antibiotics changed to meropenem 120 mg/kg/day, vancomycin 60 mg/kg/day. Despite being treated as aggressively as above, the patient’s hemodynamic status is still unstable with blood pressure 85/60 mmHg, pulse rate of 140-150 per minute. Fortunately, when we took a closer look at his medical history, we found that he had a history of COVID-19, which was diagnosed by a positive RT-PCR SARS CoV-2 test on July 24th, 2021. He had no symptoms, was concentrated quarantine with his family in COVID-19 isolation area at The Dormitory of The Vietnam National University of Ho Chi Minh City and was discharged on August 12th, 2021. After expert consultation and department discussion, we confirmed the diagnosis of MIS-C following SARS-CoV-2 infection and decided to administer intravenous methylprednisolone at a dose of 2 mg/kg/day in two divided doses and enoxaparin subcutaneously 0.5 mg/kg twice a day. Treatment results showed that the condition improved and maintained stable hemodynamics after the first dose of methylprednisolone. After 10 hours of shock management, the patient’s vital signs improved with pulse rate of 100-110 mmHg, blood pressure of 110/72 mmHg; noradrenaline and adrenaline vasopressors were stopped, and dobutamine was maintained at 5 μg/kg/min.
In the following days of treatment: The patient’s vital signs got back to the normal range, vasopressors and oxygen therapy was wean off. Other symptoms, such as fever, abdominal pain, erythema, swelling of the lips and edema of the eyelids, or edema of the hands and feet, recovered and disappeared. Further treatment was intravenous methylprednisolone 2 mg/kg/day for 3 days following 1 mg/kg/day for 5 days; then maintain oral prednisolone 1 mg/kg/day for 4 days, enoxaparin subcutaneously 0.5 mg/kg twice a day for 12 days, aspirin 3 mg/kg/day for 9 days. He was discharged on the 16th day of the illness (September 9th, 2021), and continue to take prednisolone 1 mg/kg/day plus aspirin 3 mg/kg/day as an outpatient therapy, see a follow-up appointment 2 weeks later.
3. DISCUSSION
This is the first case of MIS-C treated at Thu Duc City Hospital and in the southern part of Vietnam from starting of the COVID-19 pandemic. The diagnostic of MIS-C, in this case, was based on the WHO case definition (WHO 2020) [2]. The WHO criteria can easily be applied for diagnosis of MIS-C in low-resource settings if all the options in the criterion are followed. Cardiac injury is one of the critical features in the diagnosis and treatment of MIS-C [5-7]. In this case, we monitored the progress of cardiac enzymes (Troponin Ths, NT pro-BNP, pro-BNP), performed echocardiography every day to monitor this complication (Table 3).
Bacterial infection cannot be excluded due to the high prevalence of bacterial infections in Vietnam, high inflammatory response, and abnormal abdominal ultrasound and chest x-ray. However, the use of antibiotics for 2-3 days did not show improvement in either the clinical or inflammatory status of the patient. Some children with MIS-C may experience distributive shock, with reducing vessel myogenic tone and cardiac contractility. Epinephrine or norepinephrine are the preferred vasopressors for shock management. Milrinone may also be helpful in cases of severe reduction in left ventricular contractility [9].
The use of glucocorticoids as a sole treatment option in under-resourced settings has also been reported by Ahmed et al (2020) [10] mentioned in their report. The favorable outcome with glucocorticoid alone in this patient is consistent with that reported by Riphagen et al (2020) for cases in the UK [4]. For patients with cardiovascular damage, a combination of glucocorticoids and IVIG should be used [9, 11-15]. However, IVIG was not available during social distancing times, it is more difficult to transfer serious patients to a higher facility. We monitored the patient till hospital discharge and re-examination showed no abnormalities afterward. Evidence for IVIG therapy was proved in a series of studies, 70-95% of patients were treated with IVIG (with or without an alternative) and the majority of patients in the study group improved and restored cardiovascular function [4-7, 12, 13, 15-20]. For MIS-C graded moderate to severe, in addition to IVIG and aspirin, bolus of methylprednisolone is recommended - at a dose of 2 mg/kg/day in divided doses for three days; some life-threatening cases require a loading dose of 10-30 mg/kg/day (maximum 1 g)[14]. Compared with IVIG alone, the combination of corticosteroids and IVIG has shown more benefit across many studies, especially when heart injury [12, 13, 15]. The use of glucocorticoids for MIS-C was described in many case series. Corticosteroids were used in approximately 30 to 60 percent of patients in these series and most of them recovered rapidly [12, 13, 15, 16, 18-22]. Biological agents will be considered in cases of poor response to treatment with IVIG and glucocorticoids. The use of anticoagulants in MIS-C is unclear. In mild to moderate cases of MIS-C, prophylactic doses of enoxaparin (0.5 mg/kg every 12 hours) can be used, and in severe cases, therapeutic doses may be used [23, 24].
Conclusion
MIS-C is a rare and novel syndrome, occurring after SARS-CoV-2 infection. When a child had a history of COVID-19 admitted to the hospital with a high fever, gastrointestinal manifestations and skin rashes, and increased inflammatory response, the diagnosis of MIS-C should be considered. When intravenous immunoglobulin is not available, early appropriate use of methylprednisolone may be beneficial.
