1. INTRODUCTION
Epilepsy is a chronic neurological disease that affects individuals of all ages worldwide. In 2016, there were approximately 50 million people with active epilepsy globally, and about 74% of the patients were in rural areas in low-income countries. As stated in a population-based epidemiological study in a rural district in Viet Nam, the prevalence of active epilepsy was 4.4 per 1,000, similar to some Asian countries. Following International League Against Epilepsy (ILAE), epilepsy is conceptually defined as “a disease characterized by an enduring predisposition to generate epileptic seizures,” and an epileptic seizure is “a transient occurrence of signs and symptoms due to abnormal excessive or synchronous neuronal activity in the brain” [1]. Most people with epilepsy can be treated mainly by antiseizure drugs (ASMs). However, about 30% of the epileptic population continues to have seizures despite medication, even with new generation ASMs [2, 3]. In 2010, drug-resistant epilepsy (DRE) was defined by ILAE as a failure of adequate usage of two tolerated and appropriate ASM schedules either as monotherapy or in combination to totally control seizure [3]. Patients with DRE have increased risks of a sudden unexpected death, psychiatric comorbidities, psychosocial issues, and lower quality of life, so exploring more effective management is an essential clinical need. Some alternative treatments and medications among the DRE population include epilepsy surgery, neuromodulation, and a ketogenic diet. However, in a low-income country like Viet Nam, there are several difficulties, including funding issues, lack of expertise, and lack of facilities [4].
Acupoint embedding therapy (AET) is a kind of acupuncture treatment using a needle attached to an absorbable thread that has been used to manage various disorders, including epilepsy in Viet Nam. Depending on the type of absorbable thread used, two commonly used methods in Vietnam are catgut-embedding therapy and thread-embedding acupuncture (TEA) with polydioxanone (PDO) [5, 6]. Around the world, TEA has been widely used in Korea, China, and Taiwan for the treatment of internal disease, musculoskeletal disease, obesity, cosmetic surgery, neuropsychiatry,…etc [7]. From previously published studies, the effects of TEA in controlling epileptic seizures might come from the release of neurotransmitters including gamma-butyric acid, endogenous opioid peptides, and serotonin [8]. To the best of our knowledge, no controlled trials of the efficacy and safety of TEA in treating DRE have yet been published. The objectives of the current study were to assess the effectiveness and safety of TEA on seizure frequency and the quality of life in people with DRE.
2. MATERIALS AND METHOD
This study was a randomized, controlled, assessor-blinded clinical trial with a two-arm parallel design. Participants were recruited from the Epilepsy Clinic at the Department of Neurology, Nguyen Tri Phuong Hospital in Ho Chi Minh City, Viet Nam. Data collection was from December 20, 2020, to March 21, 2022.
We calculated the expected minimum sample size of 27 DRE patients per group with the formula:
With:
In which:
p1: the percentage of patients achieving seizure control in the control group. From the research results of the author Chen et al [2], the seizure-free control rate with 2 ASMs is 11,6% → p1 = 0,12.
p2: The percentage of patients achieving seizure control in the intervention group, expected to be as effective as ASMs monotherapy through Chen’s study [2] is 45,7% → p2 = 0,46.
r = 1.
n1: Minimum sample size for the control group
n2: Minimum sample size for the intervention group.
Unfortunately, due to the COVID-19 pandemic, only 30 patients were recruited for both groups. The inclusion criteria were: (1) adults aged from 18 to 60 years old, (2) being defined as DRE according to ILAE criteria and having one or more seizures per three later months. We excluded DRE individuals who (1) underwent epilepsy surgery; (2) had certain conditions that were inappropriate for TEA due to skin disorders (swollen, hot, and red at acupuncture points), or hemostatic disorders (international normalized ratio > 2.0 or current use of anticoagulant); (3) were pregnant; (4) had other diseases that might affect the outcomes, including body exhaustion, severe gastroenteropathy, cardiovascular disorders, diabetes mellitus, renopathy, hepatopathy, thyroidopathy, or progressive neurological signs due to acute disorders such as acute stroke, brain tumors; (5) TEA within six months; (6) current psychiatric diseases including depression, schizophrenia, or anxiety; and (7) alcoholism (more than 3 cups a day) [9].
Randomization was conducted to avoid bias and to blind the subjects and researchers. The randomness was organized at a 1: 1 ratio by lottery: (1) the treatment group with odd numbers and (2) the control group with even numbers. Statistician (T.D.P.) who was not involved in the recruitment and clinical assessment randomly assigned the participants to each group with the same probability. The independent statistician kept the generated randomization table; the file was collected from disclosure.
In this trial, blinding the practitioner (D.V.N.) was impossible due to the characteristics of the intervention. V.-D.N. is a traditional medicine doctor who performed TEA and STEA procedures; two processes were completely similar in terms of tools (needle); the only difference was that one with thread (TEA group) and one without thread (STEA group). However, it was impossible to identify which patients were undergoing TEA or STEA because after removing the needle, there was no trace of difference between TEA and STEA. However, it was designed to be assessor-blinded to control bias as much as possible. The clinical assessment was performed by a researcher who did not conduct the intervention and randomization (A.T.M.L.). A.T.M.L. is a Western medicine neurologist who did not participate in the TEA and STEA procedure and the randomization; A.T.M.L was the neurologist who performed the clinical neurological assessment to fill out the clinical information (seizure information) and the evaluation questionnaire (the NHS3) in the case report form (CRF). There was no randomization information exchange and intervention type between the two doctors (D.V.N. and A.T.M.L.).
The study included three periods: a baseline of 12 weeks, an intervention of 12 weeks, and a follow-up of the outcome data after four weeks. After the baseline period, the participants were randomly assigned to TEA or STEA through restricted randomization.
All patients were treated as outpatients and received treatment every four weeks; each patient will receive four times TEA or STEA. Each treatment lasted about 30 minutes. A Traditional Medicine (TM) doctor with ten years of experience in TEA practice (D.V.N.) performed both TEA and STEA treatments. All procedures will be performed according to the Clean Needle Technique. The methodology was based on the Revised Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement [10].
The acupoints were selected based on the Vietnamese Ministry of Health guideline [6] and based on preceding studies on acupuncture for patients with epilepsy [11-13]. The six TEA points used were GV20, BL15, BL18, ST40, GV14, and GB34. Appendix 1 shows the detailed information on TEA treatment. Among TEA group, the participants would receive a total of 4 TEA treatments every four weeks.
Mono-shaped TEA with a needle (31G-30 mm) and polydioxanone thread (7-0 USP size, 30 mm) (JBP V line; Feel-tech Co., Ltd, JBP Korea, Republic of Korea) were used in this study.
Patients were placed in a prone position. The TM doctor inserted 10 disposable sterile needles and immediately withdrew them without stimulation, and manual manipulation for Deqi would not be allowed. Before and after the treatment, practitioners sterilized the skin at the acupuncture point region with 70% alcohol-saturated cotton pads. On the day of TEA treatment, movement or stimulation of acupuncture sites was not allowed to prevent the thread from protruding.
The procedures of the Sham TEA, including acupoints and size of TEA, were similar to that of the TEA group. However, we used the thread-removed TEA in the Sham TEA group, and we performed the aseptic removal procedure of thread with secret conditions for patient blindness.
The primary outcome was (1) the mean change in seizure frequency; and (2) the quality-of-life assessment. The mean change in seizure frequency was calculated by the median number of seizures per month divided by the median number of seizures per month during baseline. The participants recorded seizure frequency using seizure diaries collected at the baseline and monthly follow-up visits. The seizure dairy was completed by patients and confirmed by caregivers because there were focal seizures in which patients still were aware during the episodes. The quality of life (QOL) assessment was recorded by The Quality of Life in Epilepsy Inventory (QOLIE-31). The QOLIE-31 was collected at the first visit for the baseline and the last visit after 16 weeks. This questionnaire included seven subscales with health concepts: emotional well-being, social functioning, energy/fatigue, cognitive functioning, seizure worry, medication effects, and overall quality of life. The Vietnamese QOLIE-31 was translated and validated. The QOLIE-31 score was calculated with higher converted scores always reflecting better quality of life. The manual guided the calculation of the total and subscale scores [14].
The secondary outcomes included (1) satisfactory seizure control was the proportion of participants with ≥ 50% reduction in seizure frequency at 16 weeks relative to baseline, including seizure freedom (no seizure recorded); (2) the National Hospital Seizure Severity Scale (NHS3) scores [15] were used to assess seizure severity at the baseline, eight weeks, and 16 weeks; and (3) epileptiform discharges (ED) were recorded by Electroencephalograph (EEG) at the baseline, and 16 weeks. Epileptiform discharges are generalized, or focal spikes and sharps followed by slow waves, from 20 - 200 milliseconds. Trained neurologists interpreted the EDs during a 20-minute standard EEG.
The researcher (A.T.M.L.) assessed the adverse events (AEs) at each visit based on vital signs, medical history, and neurological examinations. Commonly reported AEs in previous TEA studies include swelling, ecchymosis, a feeling of tightness, pain, infection, dimpling, thread extrusion, and foreign body reaction [16, 17]. We recorded all identified AEs in the case report form without referencing their association with interventions. If there were severe AEs (SAEs), the participants would temporarily stop this clinical trial.
Data were processed by Epidata 3.1 and analyzed by Stata 15.0. Quantitative variables were presented as median (interquartile range) because of skewed distributions; qualitative variables were presented as frequencies. For comparison of baseline patient characteristics, we used Mann-Whitney U-test for comparisons of medians and Fisher’s exact test for comparisons of proportions. The median difference in score changes from baseline to 16 weeks were compared between the TEA group and STEA group using Mann-Whitney U-test. Additionally, score changes for the QOLIE-31 dimensions and NHS Severity Scale were assessed for the two groups separately, using Wilcoxon signed rank-sum test. We chose a 5% significance level.
3. RESULTS
All 40 participants were screened for eligibility. Of these, ten met the exclusion criteria or withdrew consent (Figure 1).
The characteristics at baseline of subjects were not significantly different between the TEA and STEA groups (Table 1).
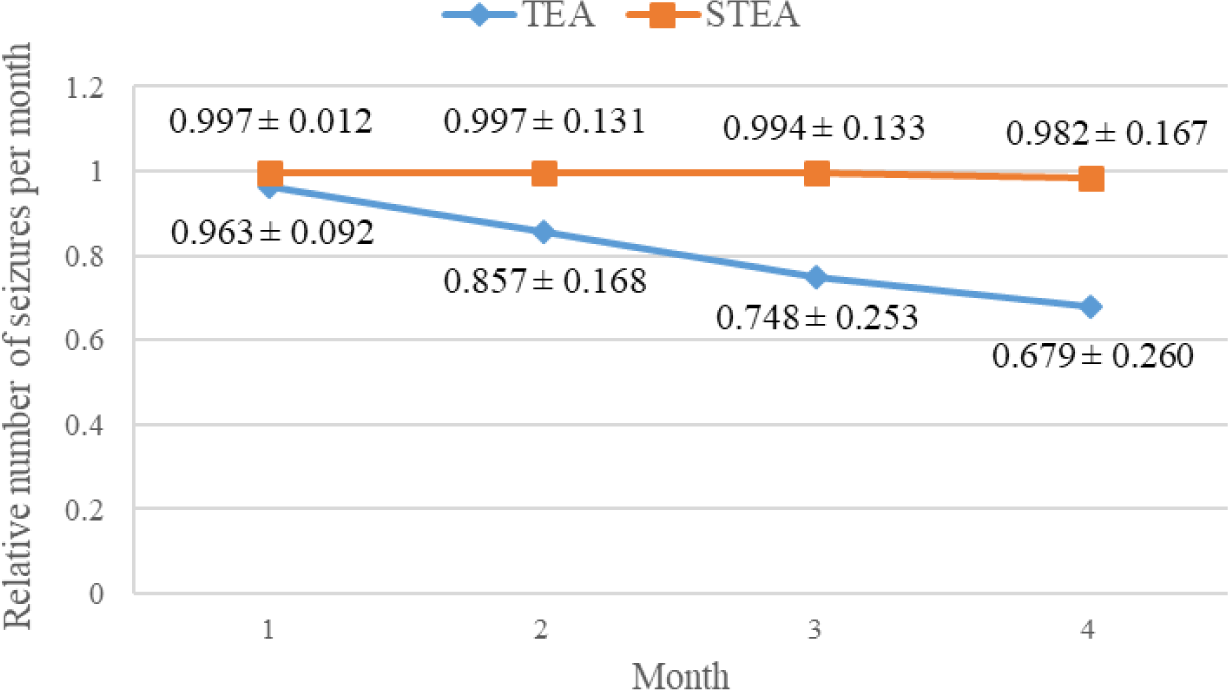
Figure 2 shows a declined trend in relative seizure frequency, i.e., the median number of seizures per month divided by the average median number of seizures per month during baseline, in both groups during this study (p<0.05, Wilcoxon signed rank-sum test).
Five participants in TEA achieved satisfactory seizure control, including one who was seizure-free, compared with one STEA patient. The seizure freedom rate and satisfactory seizure control rate after 16 weeks of study were not significantly different between the TEA group and the STEA group (p>0.05, Fisher’s exact test).
At baseline and the end of the study period of 16 weeks, no significant difference in any of the QOLIE-31 subscales and total scores was found between the TEA group and the STEA one (p > 0.05, Mann-Whitney U-test). When analysing two groups separately, significant changes from baseline to the end of the study period were seen in any of the QOLIE-31 scales except for overall quality-of-life and medication-effect subscales in the STEA group (p < 0.05, Wilcoxon signed rank-sum test) (Table 2).
No significant difference was found between the two groups in score changes on NHS Severity Scale from baseline to eight weeks. However, score changes in the NHS Severity Scale from baseline to 16 weeks as well as from eight weeks to 16 weeks in the TEA group significantly differed from those in the STEA group (p<0.05, Mann-Whitney U-test) (Table 3).
4. DISCUSSION
In this study, the seizure frequency and the score changes in QOLIE-31 and NHS Severity Scale from before of first intervention (baseline) to the end of the study of 16 weeks in the TEA group were significantly different from those in the STEA group when analyzing each group separately. From the viewpoint of traditional Chinese medicine, epilepsy is described as a disorder of disturbance of emotion and Qi. The main pathological factor of epilepsy is the retention of turbid phlegm, “no phlegm, no epilepsy” [18, 19], which results in an imbalance of Yin and Yang and dysfunction of Zang-fu (in Ancient Chinese philosophy, qi, yin, yang are abstract concepts of the energy or essence vital to homeostasis and zang-fu are used to describe the organ systems) [8, 20]. Thus, by stimulating numerous acupoints of the conception and governor vessels and twelve meridians (e.g., Heart channel, Liver channel), the disrupted flow of Qi can be adapted, and the imbalance between Yin and Yang can be restored; therefore, epilepsy is alleviated. The formula for acupoints used in epilepsy research is diverse [8]. Of the six acupoints used in this study, GV20 (to regulate liver yang, benefit the heart and calm the spirit) and GV14 (to expel pathogens and firm the yang) are the two points of the governor vessels that are widely used in acupuncture formulas for epilepsy and refractory epilepsy treatment [20]; BL15 (Back-Shu acupoints of Heart to treat heart pain and unbind the chest), BL18 (Back-Shu acupoints of Liver to expel liver wind, regulate and smoothen the qi flow and resolve depression) and GB34 (Hui meeting of the sinew; to resolve damp-heat, remove obstructions from the channel, relax the tendons and subdue rebellious Qi), ST40 (to expel phlegm and dampness) [8, 19].
Furthermore, many techniques to stimulate acupoints have been studied in treating epilepsy, such as electroacupuncture, ear acupuncture, and AET [21]. Not only may AET have a short-term effect on dredging meridians, but it also exerts long-term acupoint stimulation [5]. Catgut is a natural absorbable thread, mainly used in AET. Acupoint catgut-embedding has been used in treating epilepsy in many previous studies. Mechanisms of the effectiveness of acupoint catgut-embedding in epilepsy have been synthesized in earlier studies, including changes in nerve potentials, changes in membrane ion channels, reduction of neural circuits in seizure, induction of immune inflammation response, the release of active substances such as serotonin and arachidonic acid; reduction apoptosis number of neurons in the hippocampus by inhibiting P53 protein and increasing bcl-2 protein expression [8-18, 22]. However, PDO, a synthetic monofilament polymer made from polyester or polydioxanone polymer, is used as an alternative tool for catgut thread because PDO thread maintains similar effectiveness and optimizes adverse effects in AET [5].
DRE is a challenging issue in epilepsy treatment. The pathogenesis of DRE is complex and associated with neuroinflammatory, autoimmune, and neurodegenerative processes [23]. To our best knowledge, we are unaware of previous controlled studies reported using TEA that used PDO thread for DRE with a prospective design similar to our research. In this study, the PDO thread may have maintained similar efficacy to the catgut thread for epilepsy and exerted some effect on DRE control through the decrease of seizure frequency and the score change in QOLIE-31 and NHS Severity Scale from before of first intervention (baseline) to the end of the study of 16 weeks in the TEA group were significantly different from that in the STEA group when analyzed each group separately. PDO is a safe and well-tolerated bioresorbable polymer with no considerable adverse effects on immune functions and a tendency to induce anti-inflammatory responses with long-term exposure in vivo [24]. However, when comparing the TEA and STEA groups, there was no significant difference in the change in seizure freedom, QOLIE-31, and NHS scores. Besides, the change in EEG has not been recorded, possibly because the follow-up time is not long enough to cause a change in EEG. The adverse effects of TEA in this study were similar to those of previous TEA studies, demonstrating that TEA is a safe technique to use in patients with DRE.
There were some limitations in this study. Firstly, the sample size was small, which may reduce the power of the research and increase the margin of error. Secondly, there might have information bias during recording the seizure diaries due to unconscousness of participants. Thirdly, without individualized procedures following the Traditional Chinese Medicine syndrome, all participants underwent the process the same way, which might impact the results. Next, randomization using even and odd lottery can limit randomization and blind. In the present study, we followed up for 16 weeks (from the time of first intervention to the end of the study) in selected DRE patients who had seizures within the past 12 weeks. However, in the clinical practice of DRE treatment, the follow-up period was usually one year. Accordingly, further studies with a more extended follow-up period (one year or more) are needed to evaluate the results.
Conclusion
We demonstrated the first prospective clinical trial to assess the efficacy and safety of the TEA treatment for DRE using objective and quantitative measurements. The findings of this study can be applied as a basis for further research with modified procedures or studies, and TEA can be indicated in combination with the control of DRE in the context of a low-income country such as Vietnam, which has not yet been able to popularize epilepsy surgery, neuromodulation, and ketogenic diets.