1. INTRODUCTION
Currently, most medical diagnoses are based on test results(1). However, analytical errors at the pre-analytical period will lead to unexpected changes in analytical results in biological samples(2,3). There are several conditions where the HbA1c test can produce inaccurate results. Delays in transportation and sample handling, and whole blood samples stored at inappropriate temperature conditions can cause inaccurate test results 4-9. Therefore, the stability of biochemical parameters has been evaluated under the conditions of transporting and storing samples at the pre-test period to ensure the accuracy of test results(4-9).
Test %HbA1c has significant importance in diagnosing diabetes and monitoring the effectiveness of blood sugar control for patients(10). HbA1c levels were usually measured in laboratories using high performance liquid chromatography (HPLC) method due to its high accuracy of results. In recent years, HbA1c levels have been measured by various methods such as affinity assay, immunoassay (IA) and enzymatic assay. However, HbA1c levels may vary depending on the analytical equipment and reagents used. Although HPLC has been considered the reference method for other methods of HbA1c quantification, in practice, depending on the analytical conditions, laboratories often use analytical methods that are faster and less complex than HPLC, such as IA, to determine %HbA1c(11). HbA1c immunoassay is a bioanalytical method in which the HbA1c concentration depends on the response of HbA1c to HbA1c antibodies. The HbA1c immunoassay has been shown to correlate well, with low concordance accuracy, and with low bias with the HPLC method. However, differences in HbA1c results between immunoassays were still found(12). There have not been many studies evaluating the difference in HbA1c results between immunoassays in Vietnam. Many studies on the stability of HbA1c have been carried out, but there are no studies on HbA1c storage conditions with different quantitative methods under storage conditions in Vietnam at present. The objective of this study was to evaluate the stability of HbA1c in whole blood at different temperatures over time by two HbA1c immunoassays in order to increase the accuracy HbA1c test results, thereby to improve the treatment for diabetes patients.
2. MATERIALS AND METHOD
Erba HbA1C test reagent kit and SD Biosensor Standard F HbA1C kit for HbA1C qualitative assay were obtained from ERBA Diagnostics Mannheim and SD Biosensor, respectively. Standard F Analyzers (SD Biosensor) and Erba XL640 system (Erba Mannheim) which meet IVD certificate were used to determine HbA1c levels in human whole blood samples with respective reagent kits.
Whole blood samples for the purposes of this study were approved by the Ethics Committee of University of Medicine and Pharmacy at Ho Chi Minh City issued together with Decision No. 759/ÐHYD-HÐÐÐ October 20, 2022. Ten ml of venous whole blood from ten volunteers (5 men and 5 women) were collected in blood tubes containing K2EDTA anticoagulant and gently mixed. Whole blood was then divided into sterile test tubes (0.5 mL/tube). Blood donors who have met the criteria for blood donation according to Circular 26/2013/TT-BYT issued on September 16, 2013, such as from 18 to 60 years old, minimum weight 42kg for women and 45kg for men, and do not use any drugs. The exclusion criteria for blood samples included missing sampling information, clotted blood, cracked or broken sample tubes, and anticoagulants other than K2 EDTA.
All procedures for blood sampling, sample processing and HbA1c quantification in blood samples were carried out at Buon Ma Thuot Medical Testing Center, which has been recognized to meet ISO 15189:2012 for laboratory biosafety cabinet - Class II. The HbA1c analyzers were checked with internal control samples for HbA1c each day before HbA1c quantification in the blood samples.
Blood tubes were stored under temperature conditions such as 20-25 °C, 2-8 °C, and -20 °C to evaluate stability of HbA1c levels (%HbA1c) in whole blood. %HbA1c in whole blood samples at each time point was determined simultaneously on Standard F Analyzer and Erba XL640 system using immunoassays.
The test procedure on STANDARD F Analyzer was briefly summarized as follows: 5 μL of whole blood was placed in a tube containing extraction buffer and latex tablet and then mixed well. All specimen mixture was collected to analyze on STANDARD F Analyzer. STANDARD F Analyzer measures the color intensity of the total hemoglobin and the HbA1c assay zones separately at the test device. The percentage of HbA1c in the blood sample is the ratio between HbA1c and hemoglobin(13).
The procedure for HbA1c quantification with the Erba HbA1c kit is based on the immunoturbidimetric method. The test procedure on Erba XL640 Analyser was briefly summarized as follows: 10 μl of whole blood was lysed with 500 μl of hemolyzing solution for 5 min. After mixing 30 μl of lysate and 1 ml of buffer containing latex particles in a test tube and incubating for 5 min, 500 μl of buffer containing anti-human HbA1c monoclonal antibody and anti-mouse IgG polyclonal antibody was added. Absorbance was read accurately at 660 nm for 5 min. Erba XL640 system directly measures HbA1c levels without determination of the total hemoglobin.
At 20-25 °C, HbA1c levels in blood samples were measured at times 0 (T0), 4 (T4h), 12 (T12h), and 24 hours (T24h). At 2-8 °C, HbA1c levels in blood samples were measured on days 1 (T1d), 2 (T2d) and 4 (T4d). At -20 °C, HbA1c levels in blood samples were measured at months 1 (T1m) and 3 (T3m). HbA1c quantification was performed according to the manufacturer’s instructions. HbA1c levels in each blood sample from 10 volunteers were measured twice. Blood samples stored at 2-8 °C and -20 °C must be conditioned at room temperature for 15 minutes before HbA1c quantification and should not be reused for further testing. The stability of HbA1c was assessed by comparing HbA1c concentrations at the following time points with baseline (T0). Thus, the mean HbA1c concentrations of 10 volunteers stored at 20-25°C were quantified at 4 hours, 12 hours and 24 hours and compared with baseline (HbA1cT0). This comparison was similar for blood samples stored at 2-8°C and -20°C. If the HbA1c concentration is determined to be unstable at a one-time matter, no subsequent determination of HbA1c is necessary. The influence of the quantitative method on HbA1c was investigated by comparing the difference in the results of HbA1c quantification between the two methods at each testing time.
3. RESULTS
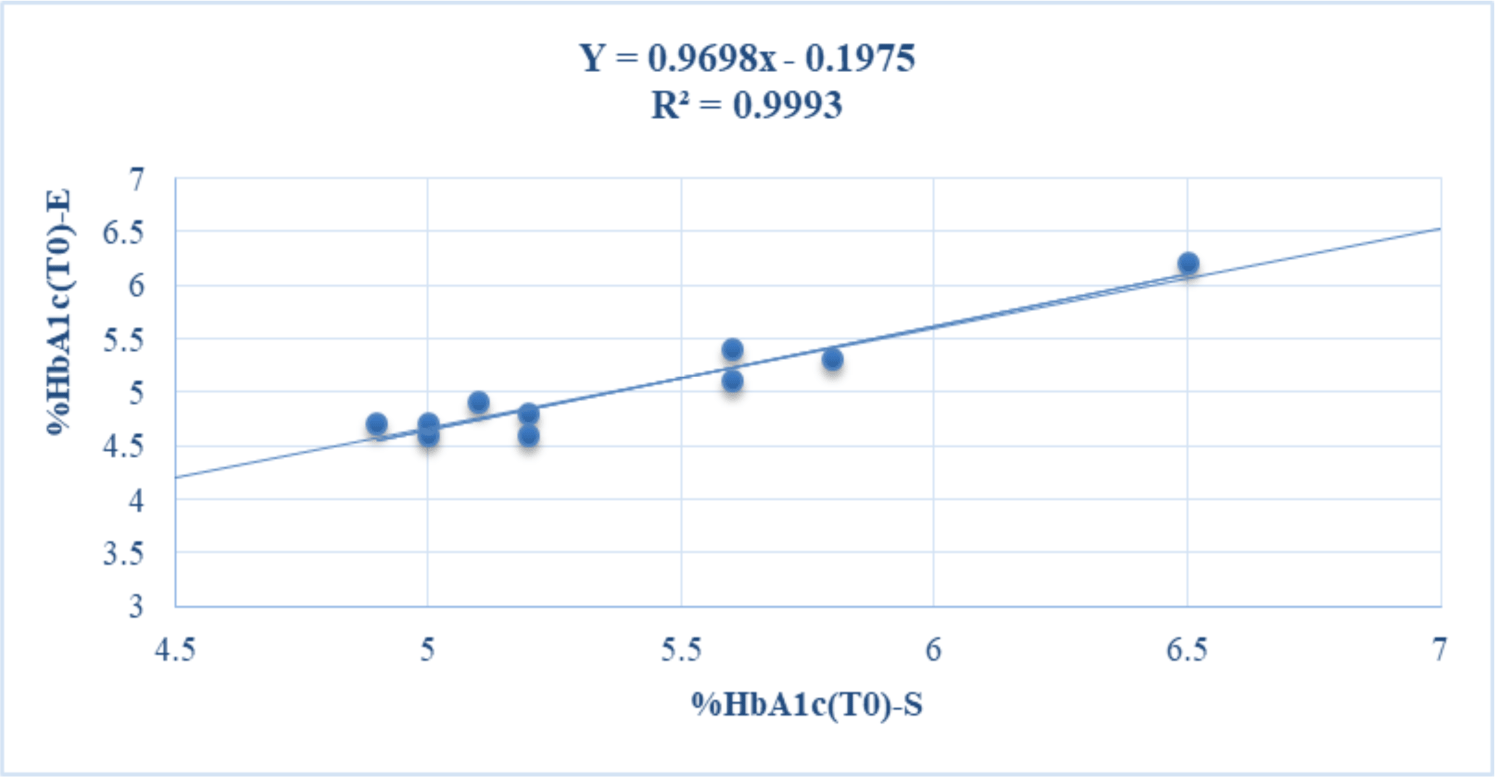
The baseline concentrations of HbA1c in whole blood samples of volunteers determined (T0) at room temperature on Standard F Analyzer (%HbA1c-S) and Erba XL640 system (%HbA1c-E) were in the range of 4.9-6 and 4.6-6.2%, respectively. Based on the results of the HbA1c test by two analytical methods, most of the volunteers in this study were healthy. Only one volunteer had %HbA1c near the threshold of 6.5%, so it is necessary to check the HbA1c level with another test method before diagnosing pre-diabetes (see table 1). The baseline levels of HbA1c-S (%HbA1c(T0)-S) and HbA1c-E (%HbA1c(T0)-E) were positively and strongly correlated with each other (r = 0.9996) (see figure 1).
The average concentrations of HbA1c of 10 volunteers stored at 20 - 25 °C were quantified at 4 hours, 12 hours and 24 hours, and compared with baseline levels (HbA1cT0). The results showed that the HbA1c-S concentrations remained stable for up to 24 hours, while the HbA1c-E concentrations were only stable for up to 12 hours compared to HbA1cT0 levels (p > 0.05). At 24h, the HbA1c-E concentrations were significantly different from the HbA1cT0 levels (p = 0.01 < 0.05) (see table 1).
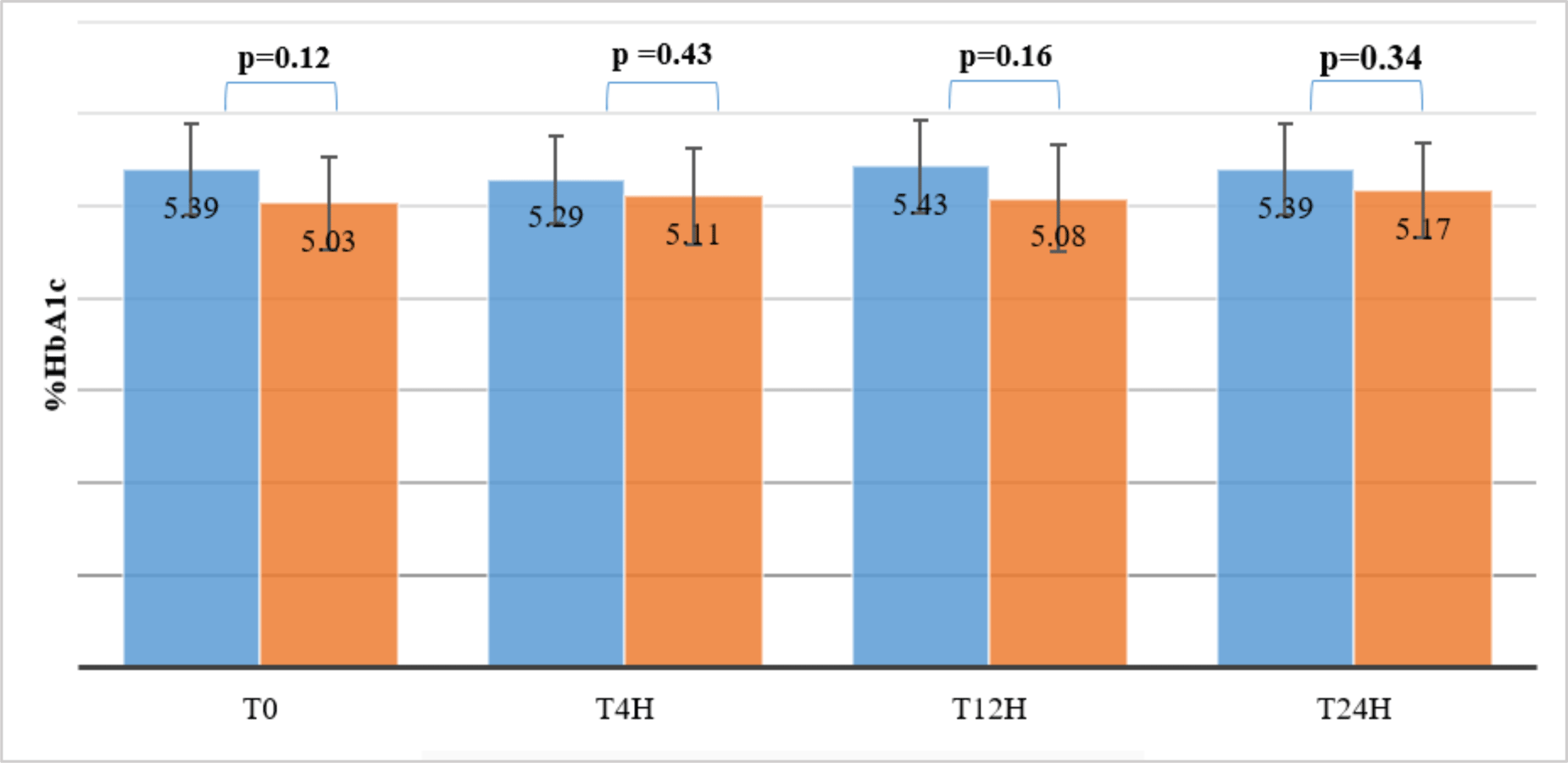
However, %HbA1c-S and %HbA1c-E determined simultaneously on the same sample stored at 20-25 °C at T0, T2h, T4h and T24h were not significantly different (p > 0.05) (see figure 2).
The average concentrations of HbA1c of 10 volunteers stored at 2-8 °C were quantified on the first, second, and forth day, and compared with baseline levels (HbA1cT0). The results showed that the HbA1c-S concentration was stable under four days, while the HbA1c-E concentration was stable for less than 24 hours compared to HbA1cT0 levels (p =0.01 < 0.05). Therefore, %HbA1c-E was no longer determined at T2d and T4d. However, %HbA1c-S and %HbA1c-E determined simultaneously on the same sample stored at 2-8 °C at T1d was not significantly different (p >0.05) (see figure 3A).
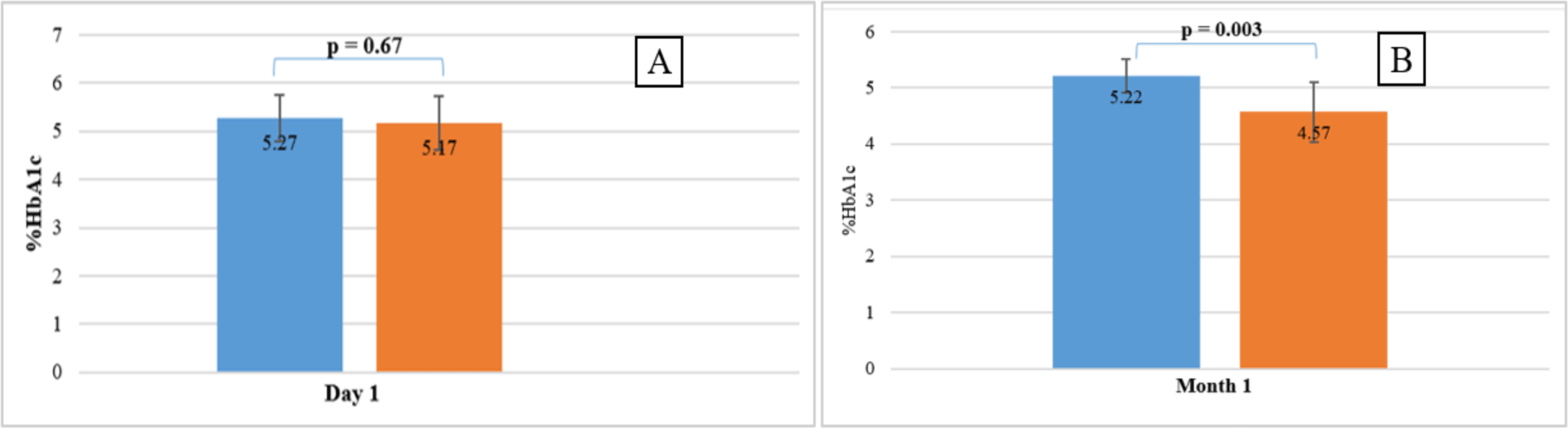
The average concentrations of HbA1c of 10 volunteers stored at -20 °C were quantified at month 1, month 3 and compared with baseline levels (HbA1cT0). The results showed that the HbA1c-S concentration was stable under 3 months (p = 0.0003 < 0.05), while the HbA1c-E concentration was stable for less than 1 month compared to HbA1cT0 levels (p < 0.0001). Therefore, %HbA1c-E was no longer determined at T3m. %HbA1c-S and %HbA1c-E determined simultaneously on the same sample stored at -20 °C at T1m was significantly different (p = 0.003 < 0.05) (see figure 3B).
4. DISCUSSION
HbA1c is one of the important indicators used to diagnose and monitor the effectiveness of diabetes treatment. Previous studies have shown that adverse sample storage conditions such as temperature changes and delayed time may affect the results of HbA1c quantification(14,15). In this study, we evaluated the influence of different temperatures on the stability of HbA1c as measured simultaneously by immunoassays on the HbA1c Standard F Analyser and Erba XL640 system.
The results showed that HbA1c-S levels were stable over 24 hours at 20 – 25 °C, under 4 days at 2 – 8 °C, and over 30 days at -20 °C. However, HbA1c-E levels were stable at under 24 hours at 20 – 25 °C, under 1 day at 2 – 8 °C and under 30 days at -20 °C. However, the average concentration of HbA1c-S at 20-25 °C and 2-8 °C at each time of survey was not statistically different from HbA1c-E at the same temperature, except at -20 °C (p < 0.05). Therefore, the HbA1c level measured on the HbA1c Standard F Analyser appeared to be more stable than on the Erba XL640 Analyser.
The baseline levels of HbA1c-S (%HbA1c(T0)-S) and HbA1c-E (%HbA1c(T0)-E) were positively and strongly correlated with each other. These results were similar to previous studies(15,16). Both immunoassay analyzers measured similar HbA1c levels at T0, which can be used to diagnose and monitor diabetes. The HbA1c immunoassays have high sensitivity, specificity, and accuracy(17). However, immunoassays that indirectly measure %HbA1c based on total hemoglobin can cause an increase in %HbA1c(18). In addition, immunoturbidimetric methods using antiHbA1c may give false results for HbA1c because of the Hb variants present in the blood sample(19,20). However, HbA1c Standard F Analyser does not cause the interference with haemoglobin variants (HbS, HbE, HbC, HbD, HbF) and carbamylated haemoglobin(13).
Vargasa et al. found a difference in HbA1c results between immunoassays(12). Moreover, Karami et al. showed a large deviation in HbA1c concentration between enzymatic assays, boronate affinity chromatography compared with HPLC(21). These deviations can be caused by the calibration of the analyzer as well as the reagents, or by the different principles conducted by the analyzers(22). In this study, we found that the stability of HbA1c assessed by the two analysers was different.
Biological samples are recommended to be stored properly and transported to the laboratory for a short period of time for analysis. In fact, blood samples for HbA1c quantification are usually stored at 4 °C so that HbA1c can be measured within 24 hours 14. However, if it is not possible to quantify HbA1c immediately or if is necessary to save samples for future studies, the results of this study suggested that blood samples for HbA1c measurements should be stored at -20 °C or lower to prolong stability of HbA1c.
This study evaluated the stability of HbA1c under the influence of storage conditions and quantitative methods. In the further studies, the effect of storage conditions on the stability of normal and abnormal HbA1c levels may continue to be evaluated.