1. INTRODUCTION
Pediatric gastric adenocarcinoma (GAC) is extremely rare and generates 0.05% of all gastrointestinal malignancies, the great majority of which are lymphomas and sarcomas [1,2]. Although children are less frequently affected by gastric adenocarcinoma, GAC is still challenging because of its highly aggressive growth pattern and more advanced stage in the diagnosis [3]. Additionally, adenocarcinoma having characteristics of a submucosal tumor (SMT) is uncommon, accounting for 0.2%-0.62% [4]. As its rarity, carcinogens, clinical features, treatment, prognosis, and prevention remain questionable, we describe two cases in children with some features similar to GAC in adults.
2. CASE PRESENTATION
A 14-year-old boy presented on 16 July 2021 with complaints of progressive increasing abdominal distension, flat stool, severe malnutrition, and no treatment before. He had lost 4kg in 5 months. His father died 11 months ago because of gastric carcinoma.
The patient was pale. Abdominal distension was attributable to massive ascites rather than hepatosplenomegaly. No cervical, inguinal, or axillary adenopathy was palpable.
Hemoglobin was decreased. Biochemistry analysis study of the ascites showed yellow, 12 cells/mm3, scattered mature lymphoid cells, lacking malignant cells, glucose 6.0mmol/L, protein 33.5g/L.
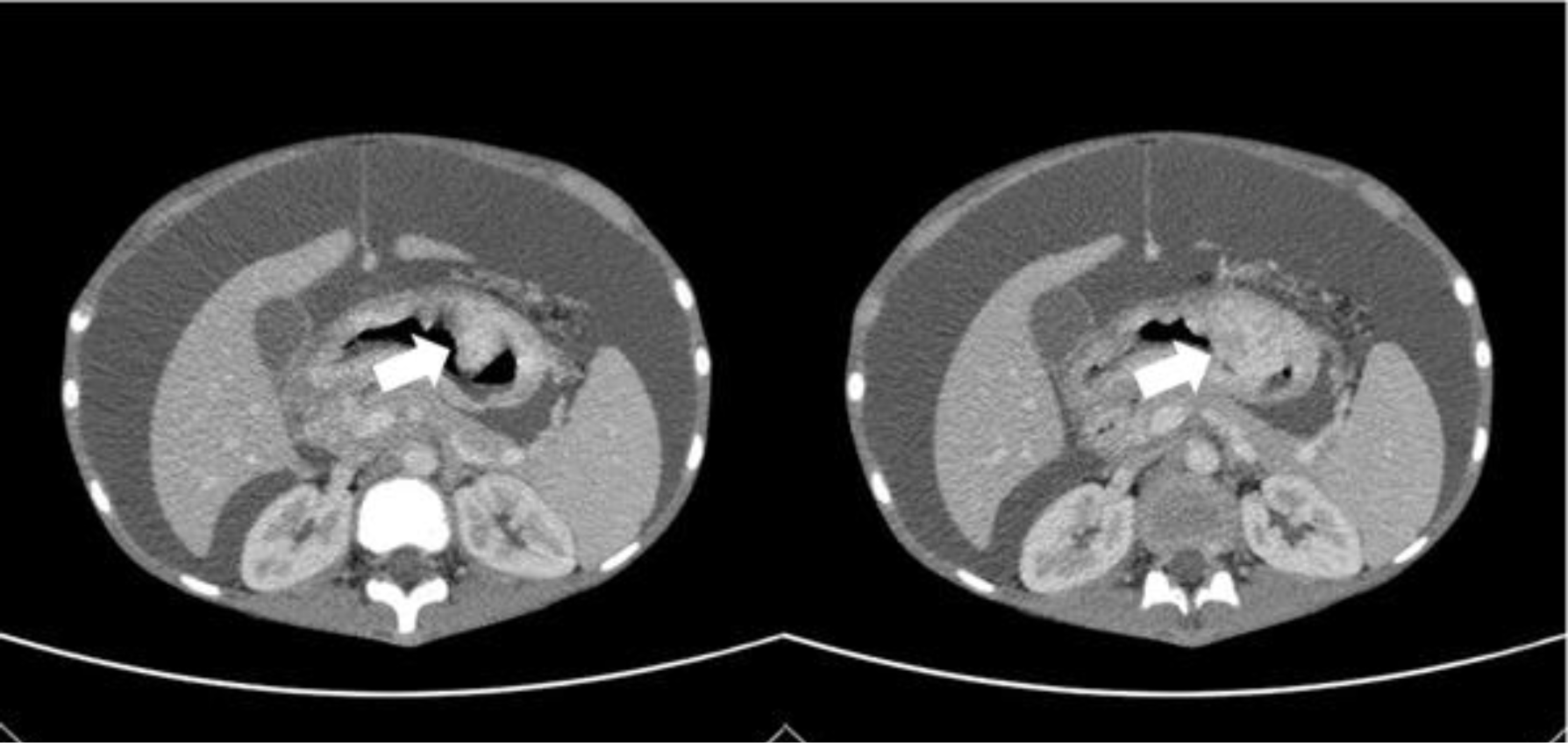
Abdominal ultrasound demonstrated abundant fluid, diffusely thick gastric wall d#12mm. The upper abdominal peritoneum was infiltrated, focally thickened, had an irregular border, and was surrounded by many large lymph nodes d#20-30mm. Computed tomography of the abdomen revealed a tumor protruding into the stomach with an irregular border associated with peritoneal metastasis and thickened gastrointestinal wall (Fig 1). Upper gastrointestinal endoscopies were performed, and gastric biopsies were taken.
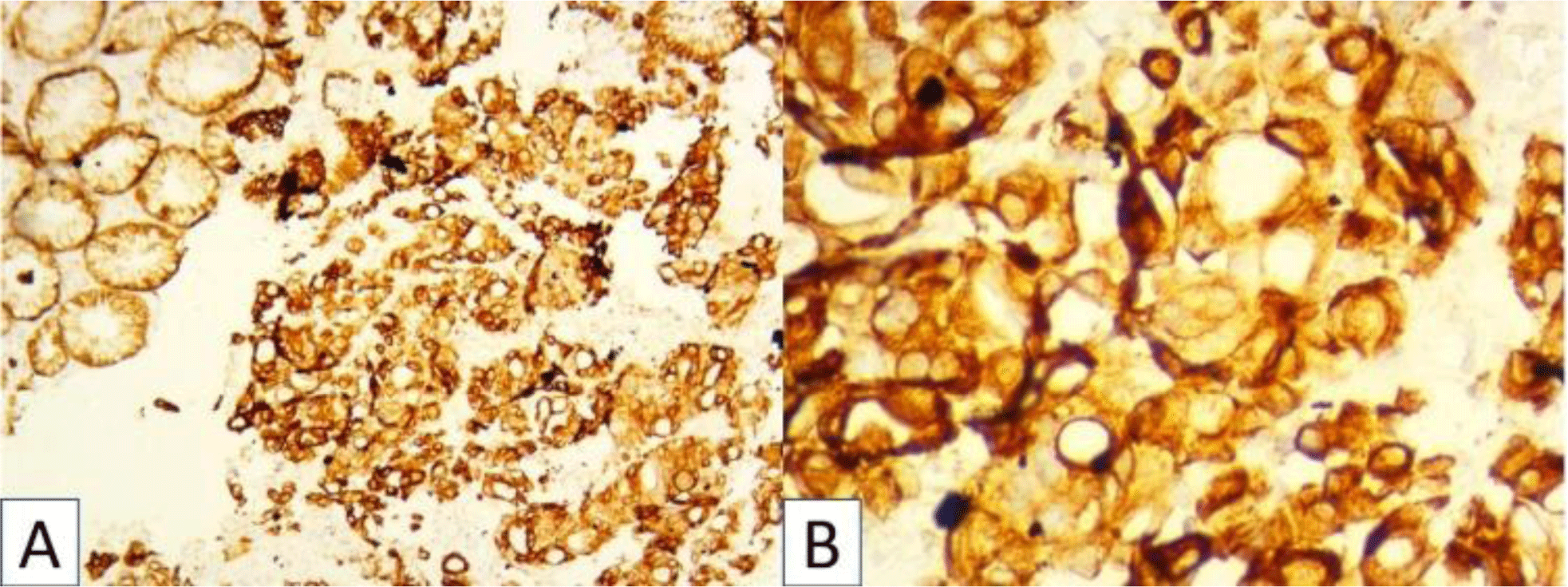
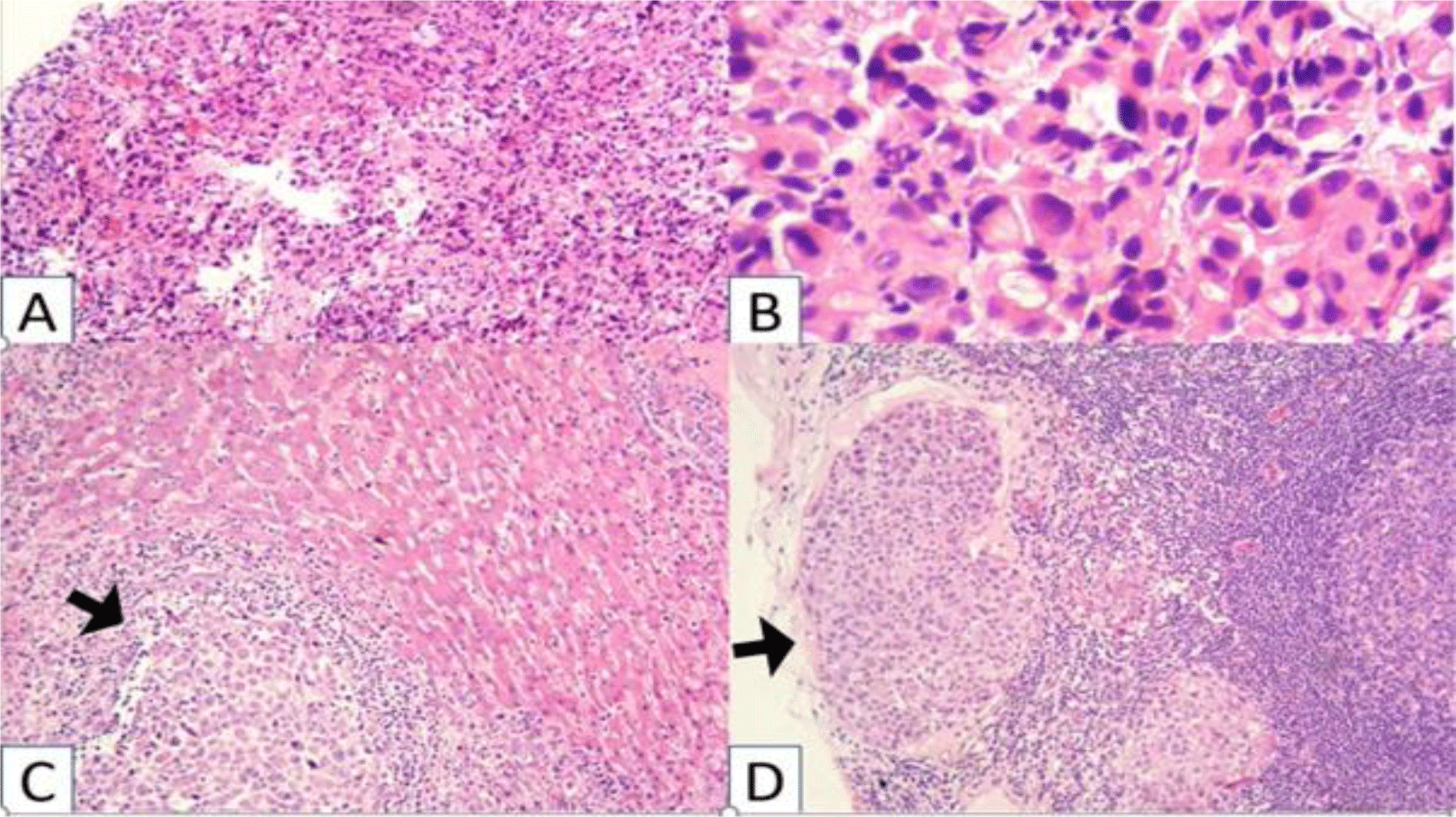
Histopathologic examination of biopsies revealed pleomorphic cells with variable sizes and increased N/C ratio (Fig 2). These cells were arranged into sheet growth patterns, clusters of cells or incomplete tubules with neutrophils infiltrating. The malignant cells showed strong expression of CK (Fig 2), negativity for CD99, NSE, Myogenin, CD3, CD20, ALK, and CD30.
His diagnosis was poorly differentiated gastric adenocarcinoma, a diffuse type.
His treatment information was missing because he had moved to another hospital for treatment.
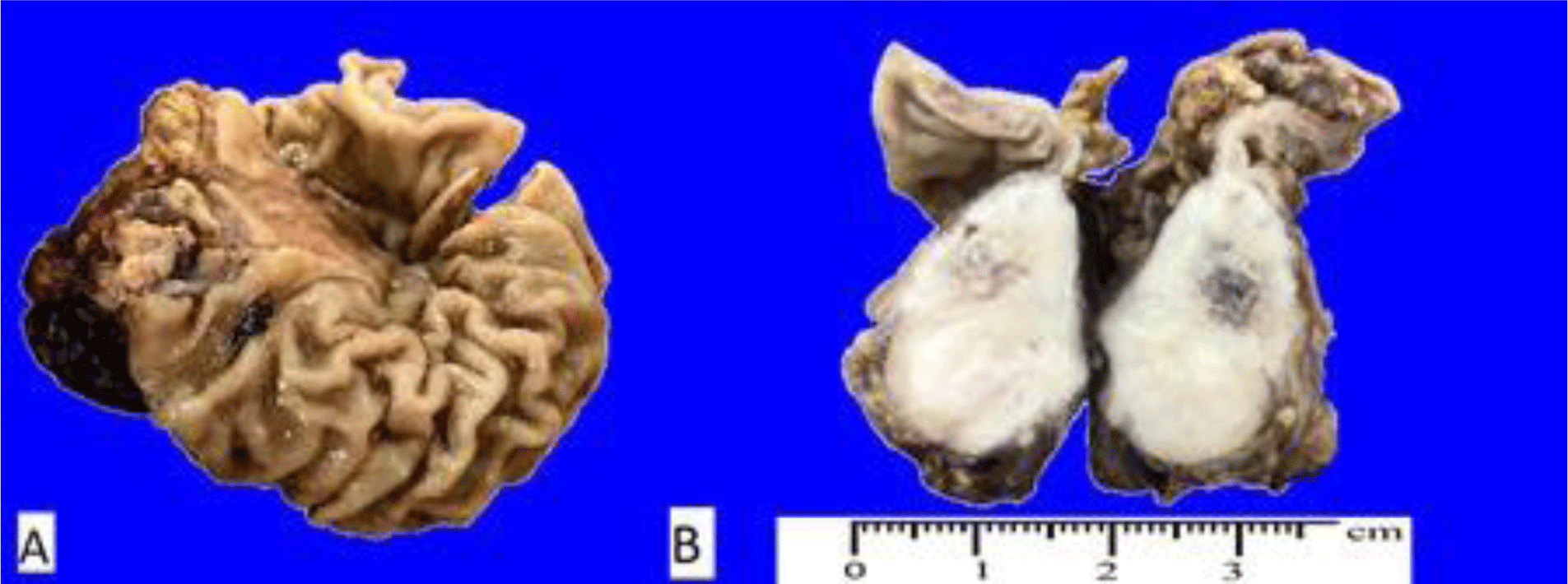
A 10-year-old boy was admitted to the hospital with abdominal pain. He had poor appetite and weight loss. His family history was unknown. Physical examination found abdominal tenderness and no cervical; inguinal adenopathy was palpable. A large tumor in the submucosa of the stomach and an abnormal node in the liver was found on CT scans. The gastric resection was taken, and perigastric lymphadenopathy was noted. The tumor was in the submucosa with white, firm, and circumscribed cut-surface, hemorrhage in the center, and infiltrating beyond the serosa (Fig 3).
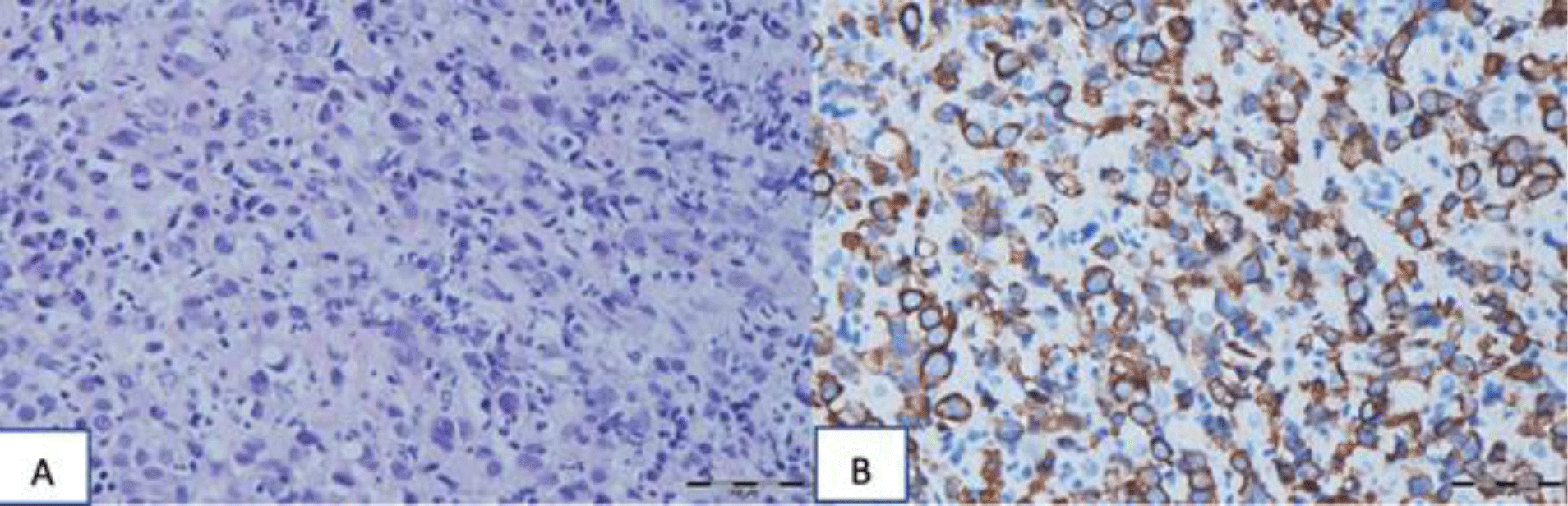
Microscopic findings showed pleomorphic cells with variable sizes and increased N/C ratio. The malignant cells are arranged discohesively with a few signet-ring cells (Fig 4). The bizarre nuclei were vesicular with prominent nucleoli. These cells were metastatic to the liver and lymph node (Fig 4). The malignant cells were strongly expressed with CK (Fig 5).
His diagnosis was poorly differentiated gastric adenocarcinoma, a diffuse type with lymphovascular invasion and liver metastasis.
His treatment information was missing because he had moved to another hospital for treatmen
3. DISCUSSION
Almost all patients diagnosed with GAC are older than 50 years [5]. Less than 5% of all new cases are diagnosed in younger patients [6]. Most gastric malignancies in children are lymphomas and sarcomas [1]. Early-onset GAC is extremely rare; only 0.05% of gastric malignancies in childhood [1,5]. Therefore, there are no criteria for diagnosing GAC in children; most cases are still based on data from adults.
At early stages, gastric carcinoma may have no symptoms; however, at advanced disease stages, it has noticeable symptoms, such as dysphagia, asthenia, indigestion, vomiting, weight loss, early satisfaction of appetite, and anemia. In case 1, the patient presented with progressive increasing abdominal distension, and the boy in case 2 was admitted to the hospital with abdominal pain. The onset of these symptoms commonly indicates advanced disease and a poor prognosis [2].
The standard gold method of diagnosing GAC is the endoscopic examination and forceps biopsies [7]. Besides, contrast-enhanced CT, MRI, EUS, or FOG PET-CT are also used for more advanced staging, notably excluding the peritoneal disease [7].
In both cases, the CT scan showed a tumor protruding into the stomach with an irregular border, especially in case 2, the cancer was located in the submucosal. Imaging examination revealed the possibility of a submucosal tumor (SMT). SMTs are non-epithelial tumors and are frequently covered with intact mucosa [4,8,9]. When SMT presents, it could be a non-neoplastic SMT, compressed by extra-gastrointestinal tumors, or a neoplastic SMT [8]. And in neoplastic SMT, there are non-epithelial and epithelial tumors [8]. Non-epithelial tumors are composed of mesenchymal tumors and lymphomas, which were frequently in children [1,8]. Epithelial tumors comprised neuroendocrine tumors and gastric carcinoma [8]. The tumor’s location plays an essential role in clinical diagnosis, and the most common tumor in the stomach is GIST [4,8]. The majority of SMT have no symptoms [8]; however, in these cases, the patients had abdominal pain and lost weight, which favors malignancy. Based on clinical signs and incidence rate, the first diagnosis of these cases was lymphoma. Although GAC in childhood is rare, this different diagnosis cannot be excluded. Therefore, pathology is essential for diagnosis.
In case 1, the boy had metastatic GAC based on a CT scan, although there was no malignant cell in the ascites because the sensitivity of this test is 56.7% [10]. Additionally, Dewitt et al (2007) revealed that 5% of patients with negative cytology were diagnosed with metastatic disease [11]. Therefore, a biopsy was taken from this patient.
Gastric carcinoma usually has poorly differentiated morphology; it is too difficult to distinguish from neuroendocrine tumors, sarcomas, or lymphomas, which are more frequent in children. In case 1, because the tumor was located under mucosa and showed no marked ulceration or raised margins, the clinician can misdiagnose the tumor as GIST, leiomyoma, or lymphoma without pathological evidence. Therefore, immunohistochemistry is critical to confirm the diagnosis, especially in these cases; CK is a valuable marker for confirmation.
In our report, both cases presented with advanced stages with peritoneal carcinomatosis (in case 1) or liver metastasis (in case 2). In case 2, on his CT scan and histology, the boy was diagnosed metastatic to the liver and lymph node. Other reports also showed that pediatric gastric carcinomas presented with metastatic diseases such as peritoneal carcinomatosis, liver metastases, and lung metastases [12]. Histopathologic characteristics of young gastric carcinoma patients are more aggressive, and most patients were metastatic at the time of diagnosis [3]. Our cases were metastatic at the diagnosis with poorly differentiated gastric adenocarcinomas, diffuse type with lymphovascular invasion.
Gastric carcinoma is a disease that has multiple causative factors [7]. About 90% of cases appear sporadically, whereas there is only 10% accompanied by inherited familial genetic components [7,13]. Patients associated with hereditary and environmental factors are at risk of developing gastric carcinoma approximately threefold [6,13]. In our case, the 14-year-old boy’s father died of gastric carcinoma; his family history played a crucial role in pathogeneses. Similarly, in Subbiah’s report, in 5 pediatric patients with GAC treated at The University of Texas M. D. Anderson Cancer Center, one patient had a family history of GAC, and another with a family history of colorectal carcinoma [5].
Additionally, pediatric cases in GAC are limited, and treatment in children, such as chemotherapy, radiotherapy, and radical surgery, remains the mimic in adults [1,14]. Besides, HER2 and E-cadherin testing give more choices for clinical application. For example, the patient with overexpressed HER2 can choose trastuzumab, targeted therapy with the anti-HER2 antibody [15]. In addition, E-cadherin is a tumor suppressor, and its expression may affect treatment and prognosis [16].
Based on reported cases of pediatric GAC, the patients in childhood may live five months after diagnosis, whereas the prognosis in adults is better; as their 5-year survival rate is 15% [2].
The children with gastric cancer were detected with advanced disease. GAC mimicking a submucosal tumor is often difficult to be diagnosed, even misdiagnosed with other tumors of these kinds.
Conclusion
Pediatric GAC presents with a more advanced stage and poor differentiation. However, the clinical presentation is like adult GAC. Because of data limitations, the diagnosis and treatment of pediatric GAC remain a significant challenge. Its presentation on CT scan is similar to SMT, such as mesenchymal tumors or lymphomas. It is essential to combine pathological and clinical examination to diagnose. In children older than ten years old who have malnutrition, gastric tumor, or multiple lymph nodes, carcinoma should be considered despite its rarity. Interestingly, choosing appropriate markers is essential in detecting this disease.