1. INTRODUCTION
Enteric duplications are rare congenital mass lesions found throughout the alimentary tract with the incidence of 1 in 4500 autopsies [1, 2]. They are formed between the 4th and 8th weeks of gestation but their etiology is still unknown [3]. These lesions were first described in the 18th century [1]. Since the article of William Ladd in 1937 [4], the term duplications of the alimentary tract has been used for those anomalies having three characteristics: a well-developed coat of smooth muscle, an epithelial lining representing some type of intestinal tract mucosa and intimate anatomic association with some portion of the gastrointestinal tract [1, 2]. Several cases of enteric duplications without any attachment to the alimentary tract, they were called “isolated enteric duplication” (IED), have been reported [3, 5-21]. We present the first case of an IED cyst which was located in the transverse mesocolon and review the literature regarding the clinical aspects of this anomaly.
2. CASE REPORT
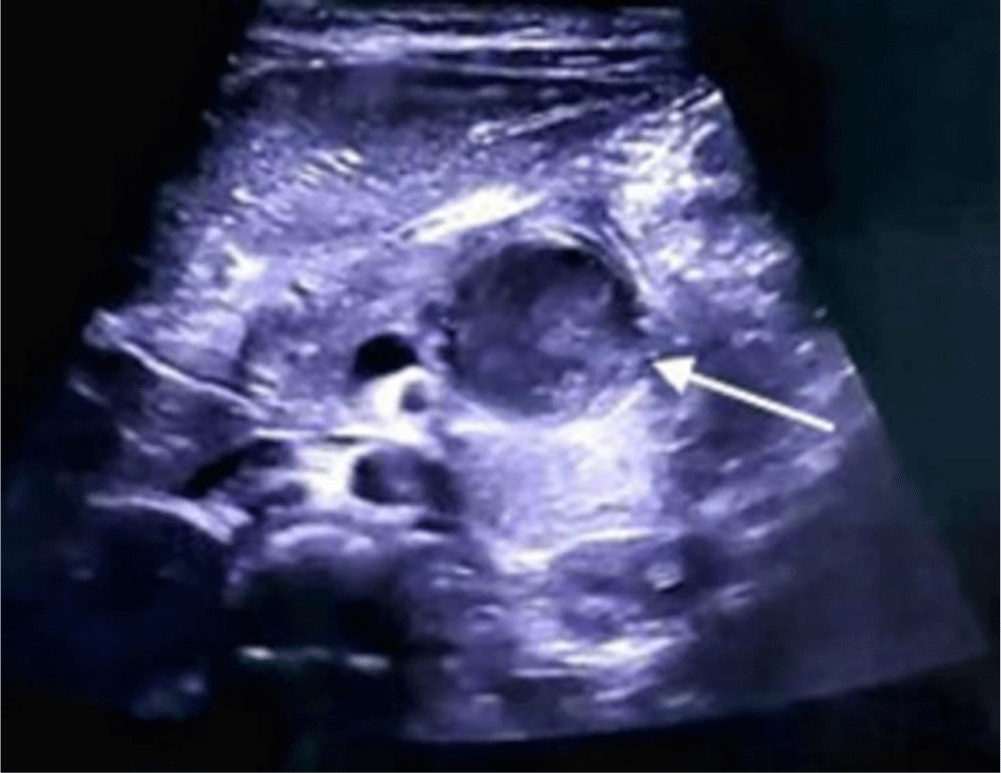
A 3-year-old girl with no remarkable past medical or surgery history presented with recurrent dull pain in the epigastric area for almost one year and non-bilious emesis for 1 day. She had not received any medical care for this situation. On examination, the girl was fully conscious and had normal vital signs. Her abdomen was soft, neither distended nor tender and no mass was palpable. Laboratory investigations showed liver function, pancreatic enzymes and other hematological tests were within normal ranges. Abdominal ultrasound revealed a retroduodenal cystic lesion measuring 35 mm × 23 mm with thick wall resembling the gastrointestinal wall. The lesion contained fluid of increased echogenicity suggesting hemorrhage (Figure 1).
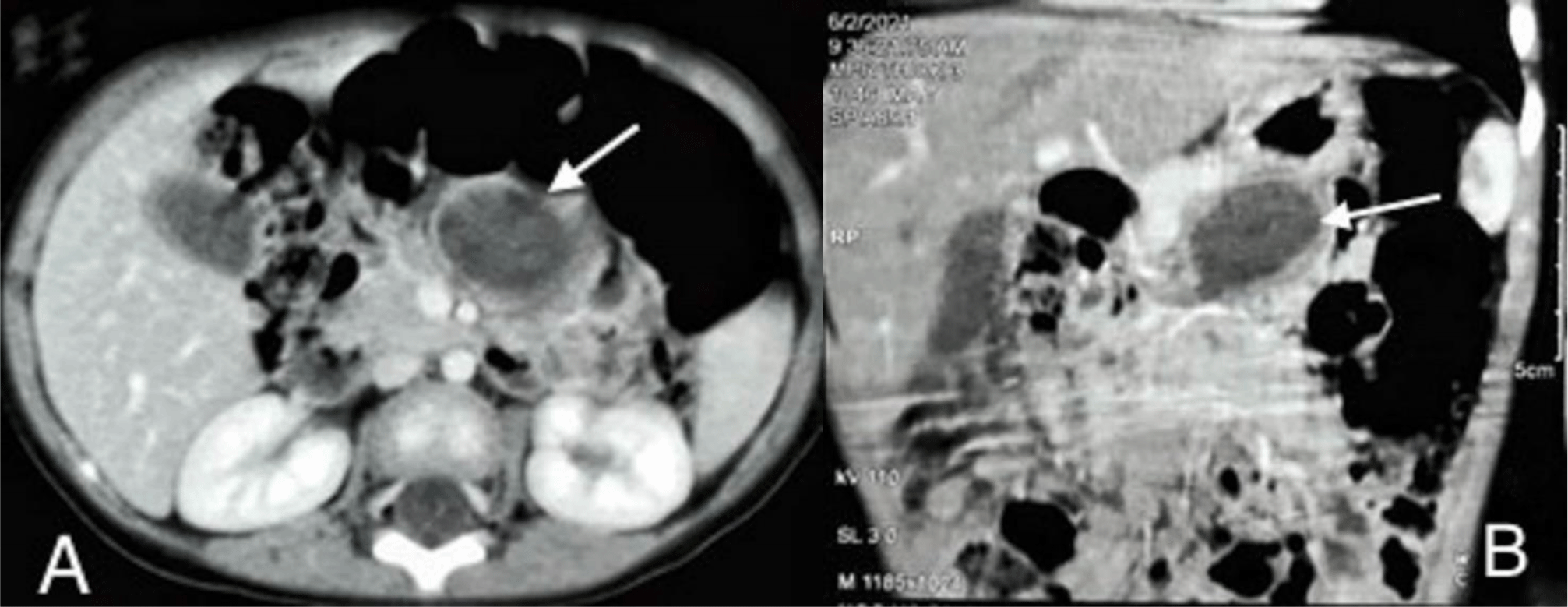
Contrast-enhanced abdominopelvic computed tomography (CT) scan showed a well-limited cyst, which has an enhancing thick wall, measuring 23 mm × 29 mm × 34 mm.
The lesion was located around duodenojejunal flexure, anteroinferiorly to the tail and body of the pancreas. The lesion was of uniformly low attenuation in the center (Figure 2A and 2B). A duodenojejunal duplication cyst or a pancreatic pseudocyst was then suspected.
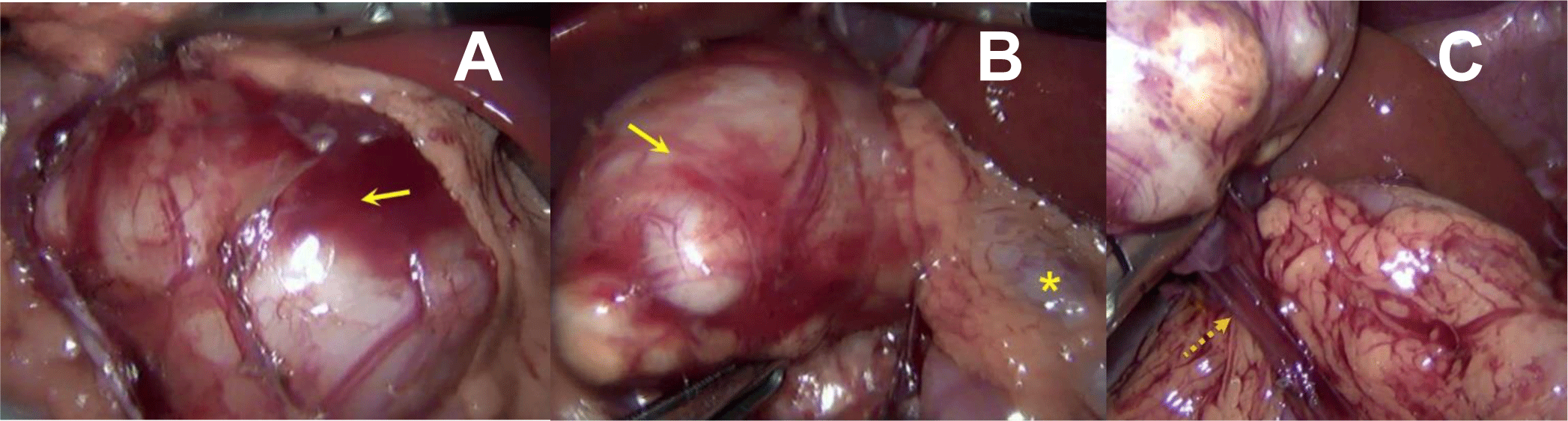
At laparoscopy, a mobile cystic mass was found on the mesentery of the transverse colon in the vicinity of the duodenojejunal flexure (Figure 3A and 3B). The intestinal tract and the pancreas didn’t have any connection with it. The mass was completely dissected free of bordering soft tissue without interrupting any normal intestinal or mesenteric structure. Its dedicated vascular pedicle originating from the transverse mesocolon and was simply cauterized and removed (Figure 3C).
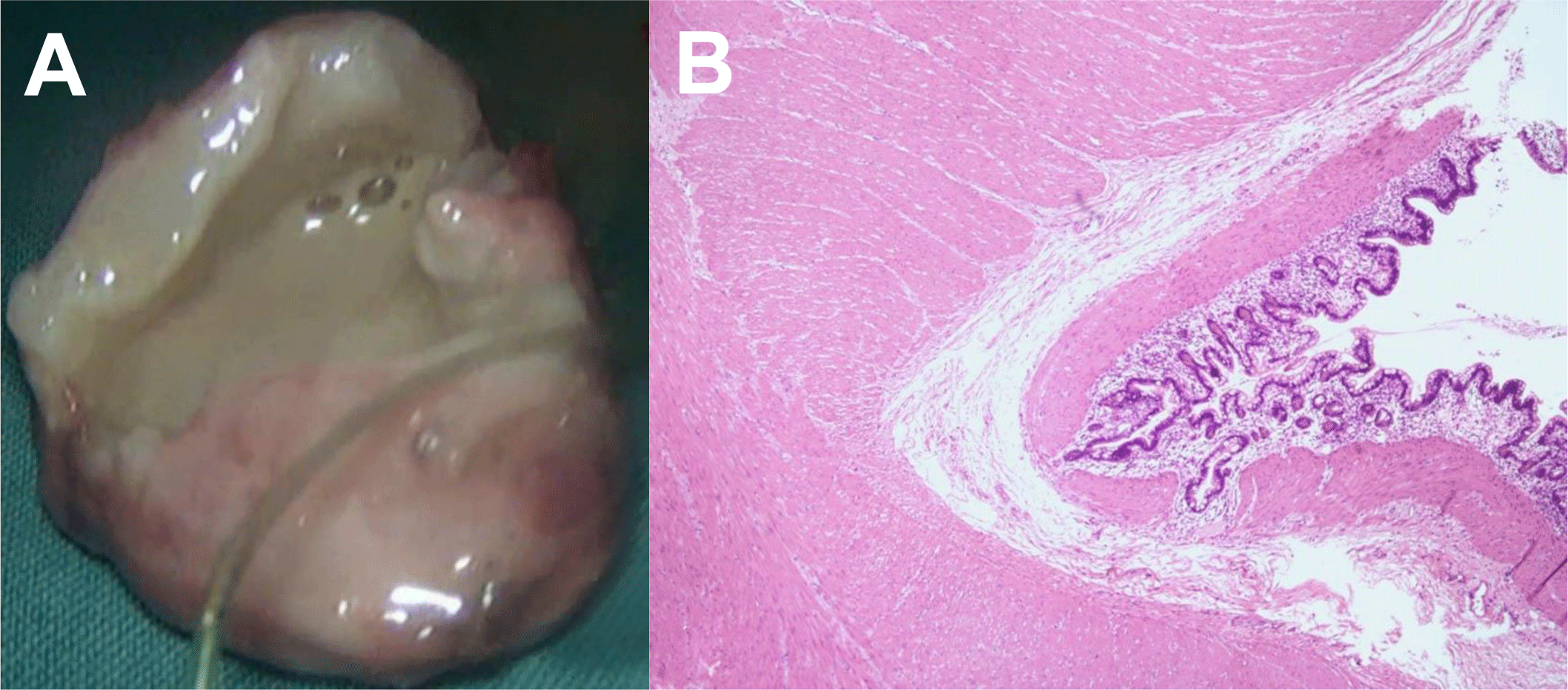
The resected specimen had dimensions 3 cm × 3 cm × 2 cm, tense and suppurative. On cut section, the mass contained purulent yellowish-grey fluid within a thick wall (up to 0.5 cm) (Figure 4A). Histopathology of the cyst wall showed well-developed smooth muscles, connective tissue and gastric mucosa, which was the microscopic identity of enteric duplication cyst (Figure 4B).
Postoperatively, the girl was given oral feeding when she was fully alert and was discharged without any complications on the second day after surgery.
3. DISCUSSION
Alimentary tract duplications are uncommon malformations occurring anywhere from the mouth to the anus; most commonly in the ileum (33%), followed by the esophagus (20%), colon (13%), jejunum (10%), stomach (7%) and duodenum (5%) [2, 3]. The variety of duplication forms and associated anomalies in the vertebral column, spinal cord, genitourinary system and gastrointestinal tract suggest a multifactorial process in their development [2]. A number of theories have been put forward to explain their pathophysiology, but no single theory accounts for all variants [1, 3]. Enteric duplications can be spherical (or cystic) or tubular, as well as single or multiple [1, 3]. The small duplications were also classified into two types originated in the relation of the vascular supplies to the duplication and concerned bowel: type 1 or parallel type (75.4%) and type 2 or intramesenteric type (24.6%) [22]. Enteric duplications present in a diversity of manners depending on their size, location, adjacencies, and the presence of heterotopic mucosa [1]. They can be found incidentally or by symptoms including abdominal pain, distention, obstruction, perforation, respiratory compromise or as a painless mass [2].
Regardless of the clinical manifestation, three conventional diagnostic criteria proposed by William Ladd have widely been accepted: (1) a well-developed coat of smooth muscle, (2) an epithelial lining containing the mucosa of the intestinal tract and (3) a close attachment to the digestive tract (usually share a common wall) [1-3, 6]. Many cases of enteric duplications reported were not connected to any part of the intestinal tract, they were called “isolated enteric duplication” [3, 5-21], which is similar to our present case. In clinical, the surgeon can misdiagnosed IED with other diagnosis (e.g. pancreatic pseudocyst, mesenteric tumor, etc.). The IEDs were definitively diagnosed on their histopathology after surgery.
Although most of patients with the enteric duplication are diagnosed in the first 2 years of life [1, 5, 9, 10, 14, 17, 19], it has also been detected at the age of 3 (our patient) as well as older children [6, 20, 23] and adults [8, 11, 12, 15, 16, 18, 21]. Like “typical” enteric duplication cysts, IEDs also have a wide range of manifestation according to their properties and the surrounding organs. Mandhan reported a 2-day-old boy who was diagnosed antenatally with three intra-abdominal cystic lesions near to the stomach on US [9]. Another patient had hematuria and dysuria because the cyst was communicating with the urinary tract [5]. Some patients presented abdominal distension or palpable lump and even obstructive symptoms if the lesions were too big [6, 10, 12, 17, 19]. IEDs can cause acute or chronic anemia because of bleeding [5, 17]. Furthermore, IEDs can be found incidentally by routine medical checkup or by symptoms of other comorbidities [8, 18]. Asymptomatic cysts may be the site of cancer during adult life [11, 23]. Overall, abdominal pain, which can be dull or intensive, was the most common symptom and may be associated with nausea or vomiting, especially when torsion of the cyst happened [14, 15, 21]. In our patient, the pain occurs in the epigastric area, but it can be anywhere else depending on the location of the lesion [6, 16, 20, 23].
At operation, the lesion of our patient was found close to the Treitz angle, with its vascular pedicle arising from the transverse mesocolon. Retroperitoneum and ileal mesentery seem to be the dominant sites for IEDs, followed by the vicinity of Treitz ligament, mesentery of the descending colon and jejunal mesentery (Table 1). Deng found 22 more cases of IEDs, along with his case, associated with an accessory pancreatic lobe and could therefore cause acute pancreatitis [6]. Most of the IEDs were single but in extremely rare case, multiple IED cysts existed in different sites [9, 10]. As far as we know, our patient was the first to have IED cyst on the mesentery of the transverse colon.
| Reference | Year | Age | Sex | Symptom(s) | Site | Lining epithelium |
|---|---|---|---|---|---|---|
| Ladd [4] | 1937 | 19m | M | Dyspepsia, vomiting | Terminal ileal mesentery | Simple and pseudostratified columnar epithelium |
| Duncan [7] | 1992 | 7d | (-) | Prenatal diagnosis | The left adrenal gland | Gastrointestinal mucosa |
| 1d | M | Prenatal diagnosis | Retroperitonum posterior to the pancreatic head | Intestinal mucosa | ||
| Steiner [19] | 1999 | 7d | M | Prenatal diagnosis | Mesentery in the vicinity of the Treitz ligament | Gastric mucosa, focal squamous metaplasia |
| Kim [8] | 2003 | 28y | M | Abdominal mass | Mesentery in the vicinity of the Treitz ligament | Gastric mucosa |
| Menon [10] | 2004 | 6d | M | Abdominal distension | Terminal ileal mesentery | Gastrointestinal mucosa |
| 10w | M | Abdominal mass |
(1) Mesentery in the vicinity of the duodenojejunal flexure; (2) Ileal mesentery |
Gastrointestinal mucosa | ||
| Sinha [17] | 2006 | 4d | F | Abdominal mass | Ileal mesentery | Gastric mucosa |
| Okamoto [13] | 2008 | 27d | M | Prenatal diagnosis | Retroperitonum posterior to the pancreatic tail and stomach | Gastric mucosa |
| Srivastava [23] | 2008 | 3y | F | Abdominal pain and mass | Terminal ileal mesentery | Gastrointestinal mucosa |
| Nichols [12] | 2011 | 27y | F | Abdominal mass | Descending colonic mesentery | Columnar mucinous epithelium |
| Pant [14] | 2012 | 18m | M | Abdominal pain, distension and bilious vomiting | Terminal ileal mesentery | Gastric mucosa |
| Bal [5] | 2014 | 9m | M | Haematuria, dysuria and anaemia | Retroperitonium, communicating with the right pelviureteric junction | Gastric mucosa |
| Mandhan [9] | 2014 | 2d | M | Prenatal diagnosis |
(1) Proximal jejunal mesentery (2) Retroperitoneum |
Small bowel mucosa |
| Park [15] | 2014 | 36y | F | Abdominal pain | Terminal ileal mesentery | Simple cuboidal-to columnar mucous epithelium and partly gastric mucosa |
| Sasaki [16] | 2018 | 19y | M | Abdominal pain | Retroperitoneum, between the pancreatic body and transverse mesocolon | Intestine-like mucosa and partly simple columnar epithelium |
| Xiao-Ming [21] | 2018 | 20y | M | Abdominal pain, vomiting, nausea and abdominal distention | Ileal mesentery | Transition of squamous and glandular epithelia, gastric mucosa and intestinal metaplasia |
| Nakashima [11] | 2019 | 43y | F | Abdominal pain and mass | Pancreatic head | Gastric mucosa, adenocarcinoma |
| Tanaka [20] | 2019 | 5y | F | Abdominal pain | Ileal mesentery | Intestinal tissues and pancreatic tissue |
| Deng [6] | 2019 | 10y | M | Abdominal pain | Tail of the pancreas | Gastrointestinal mucosa and pancreatic tissue |
| Siragusa [18] | 2020 | 26y | F | Dysmenorrhea | Ileal mesentery | Pseudostratified ciliate epithelium |
Total excision of the lesion was the treatment of choice because it can not only relieve the symptoms but also prevent malignant transformation [6]. Most of the IEDs can be safely removed without interfering the bowel continuity due to dedicated vascular [21, 23]. On the other hand, some cases required resection of adjacent organs for their tight connection [11]. Although the consensus has not been reached on treatment of asymptomatic duplication cyst, surgery is recommended management because the variety of the possible complications may occur in adult life [15]. Laparoscopy should be considered for diagnosis and excision since it is minimally invasive and brings preferable outcome [12, 16].
Histopathologic detections of distinct muscle wall layers and an epithelial lining, are crucial in diagnosing both “typical” enteric duplications and IEDs. Our patient’s IED had gastric mucosa, which is the most prevailing type of epithelial lining [21]. Other mucosal types existing in the IEDs include columnar epithelium, small intestinal mucosa, colonic mucosa, or mixed type [15, 16, 18, 21]. Dysplastic change and malignant transformation may arise in adults since the ectopic mucosa present and stimulate persistently [11, 21].
Conclusion
IED cyst is an unusual phenomenon with various manifestations regarding its characteristics and comorbidities. Histopathology plays a major role in definitive diagnosing and gastric mucosa is the dominant type of epithelial lining. Total resection of the cyst without injuring the digestive tract is possible due to no connection between them. The laparoscopic surgery was the preferred choice and its results brought good benefits.