1. INTRODUCTION
Critically ill patients frequently encounter increased chest wall weight and elevated pleural pressures, often resulting from multiple factors such as edema, pleural effusions, increased abdominal hypertension, and other causes [1,2]. These conditions can lead to al-veolar derecruitment, increased lung elastance, and subsequent hypoxemia in Acute Respiratory Distress Syndrome (ARDS) [1]. A major challenge in ARDS treatment is effectively titrating positive end-expiratory pressure (PEEP) to align with the physiological char-acteristics of each patient. Transpulmonary pressure, reflecting the mechanical stiffness of the chest wall, may accurately represent the shear stress applied to the alveoli injuries [3]. Positive transpulmonary end-expiratory pressure (PL-exp) has been evidenced to improve blood oxygenation and respiratory mechanics in previous studies [4,5]. However, titrated PEEP guided by esophageal pressure has been less frequently studied in Vietnamese populations, limiting clinicians ability to make individualized PEEP adjustments for ARDS patients. Therefore, our study aims to determine the incidence of positive PL-exp in the initial PEEP setting and identify factors related to adjustments of PEEP-guided by positive PL-exp to optimize and individualize PEEP settings in ARDS patients.
2. MATERIALS AND METHODS
We collected data from all patients aged 16 and above who were admitted to the mixed Intensive Care Unit (ICU) of Cho Ray Hospital, a tertiary hospital in Vietnam. Our study, conducted from November 2021 to October 2023, focused on patients diagnosed with moderate to severe ARDS according to the Berlin Definition criteria [6]. Exclusion criteria encompassed various conditions such as contraindications for esophageal pressure catheter placement, receipt of extracorporeal membrane oxygenation or prone position ventilation, severe coagulopathy, history of lung transplant, history of chronic obstructive pulmonary disease, presence of an active bronchopleural fistula, neuromuscular disorders, severe coagulopathy, pulmonary embolism, lung transplants, absence of adequate tools, or a declined to participate in the study, see Fig. 1.
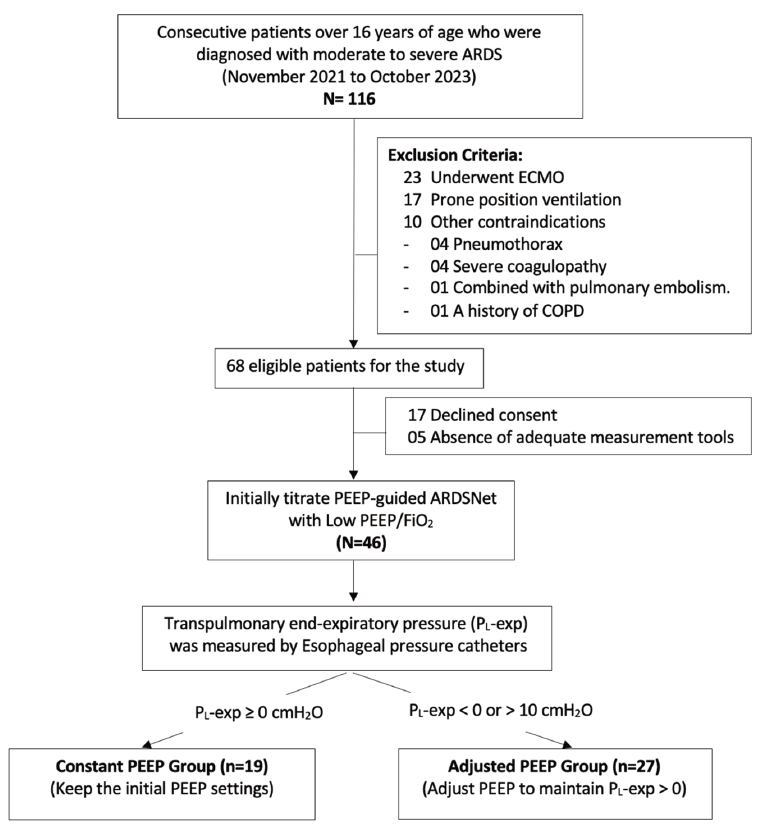
Patients eligible for the study were subjected to mechanical ventilation using the ELISA 800 Ventilator (Löwenstein, Germany) un-der the ARDSNet protocol (Table 1), targeting optimal respiratory function [7]. The ventilation strategy involved low tidal volumes set at 6–8 mL/kg of predicted body weight (PBW), while maintaining the plateau pressure (Pplat)≤30 cm H2O. In scenarios where the plat-eau pressures>30 cm H2O, a reduction in VT to as low as 4 mL/kg PBW was implemented, subsequently establishing a plateau pres-sure threshold of 35 cm H2O. The protocol set the arterial oxygen saturation target within the range of 88%–95%, or partial pressure of arterial oxygen (PaO2) between 55–80 mmHg. Arterial pH of 7.30 to 7.45, respiratory rates were limited to a maximum of 35 breaths per minute. Adjustments in PEEP were meticulously managed to optimize oxygenation while minimizing the risk of adverse hemody-namic effects. An esophageal pressure balloon (Nutrivent™, Mirandola, Italy) was inserted, and patients were sedated and given mus-cle relaxations when necessary. This facilitated the measurement of esophageal pressure (Pes) and enabled the calculation of transpulmonary pressure using the formulae: Transpulmonary end-inspiration pressure (PL-insp) = Plateau pressure – Pes-insp, and transpulmonary end-expiration pressure = PEEPtotal – Pes-exp. Patients were divided into two groups based on PL-exp: the constant PEEP group (PL-exp>0 cm H2O) and the adjusted PEEP group (PL-exp> 10 or <0 cm H2O). Finally, PEEP was modified to achieve a PL-exp minimal range of 0–10 cm H2O. Intrinsic PEEP levels were monitored before and after each adjustment, and the inspirato-ry/expiratory ratio was carefully regulated to avert the presence of auto-PEEP.
We recorded patient demographics including age, sex, body mass index (BMI), ARDS risk factors, and scores including Sequential Organ Failure Assessment (SOFA) score, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Vasopressor Index Score (VIS), and Radiographic Assessment of Lung Edema (RALE) score at ICU admission. Respiratory mechanics parameters were also collected encompassing airway driving pressure (DP), plateau pressure (Pplat), transpulmonary end-expiratory/inspiratory pressure, esophageal end-expiratory/inspiratory pressure (Pes-exp/insp), and respiratory system compliance (Crs). The primary outcome was the incidence of positive PL-exp with secondary outcomes included ventilator days, hospital stay duration, and in-hospital mortality.
Categorical variables are expressed as numerical data and percentages, and their analysis was conducted using Fisher’s exact test. Prior to analysis, continuous variables were assessed for normality using the Shapiro-Wilk test. Variables with a normal distribution are presented as mean±SD, while those non-normally distributed variables are reported as the median (25th–75th percentile). Comparisons of continuous variables between the Constant PEEP group and the Adjusted PEEP group were conducted using student’s t-test or the Mann-Whitney U test, as appropriate. Risk factors associated with adjusting PEEP were explored through both univariable and multi-variable logistic regression, with odds ratios (OR) and 95% confidence interval (CI) estimated. All statistical analyses were performed by R 3.6.2, and a two-sided p-value<0.05 was considered statistically significant.
The study was approved by the Institutional Review Board of Cho Ray Hospital’s Ethics Committee in Biomedical Research (Ap-proval Number 1229/GCN-HĐĐĐ) on November 3, 2021. All participants written informed consent or their legally authorized repre-sentatives. The study was conducted in adherence to the ethical principles of the Declaration of Helsinki.
3. RESULTS
Our cohort comprised 46 patients, with a mean age of 49.8 years, and a male predominance of 69.6%. The mean BMI was 24.7 kg/m². The majority of patients (76.1%) were classified as having moderate ARDS (PaO2/FiO2<200), while 23.9% had severe ARDS (PaO2/FiO2<100). Common observed risk factors for ARDS included pneumonia (39.1%) and sepsis or septic shock (37.0%). Lung contusion and pancreatitis each accounted for 8.7% of cases, with four patients presenting with each condition. Other contributing factors comprised 6.5% of cases. Respiratory mechanics revealed a median plateau pressure of 27.0 cm H2O [IQR 24.0–29.0], airway DP of 17.0 cm H2O [IQR 14.0–20.0], and respiratory system compliance (Crs) of 23.8 mL/cm H2O [IQR 19.7–27.7], as summarized in Table 2.
Data are presented as n (%) for categorical variables and the median (interquartile range) for nonparametric variables.
APACHE-II, Acute Physiologic Assessment and Chronic Health Evaluation-II; BMI, body mass index; CCI, Charlson Comorbidity Index; Crs, compliance respiratory system; SOFA, Sequential Organ Failure Assessment; FiO2, fraction of inspired oxygen; PaO2, partial pressure of arterial oxygen; PEEP, positive end-expiratory pressure; VIS, Vasopressor Index Score.
In our study, patients were stratified into two distinct group based on the PEEP-guided PL-exp: a Constant PEEP group (comprising19 patients) and an Adjusted PEEP group (comprising 27 patients). The findings of our study indicated that the incidence of positive PL-exp at 41.3% (19 out of 46 patients) and 58.7% of the patients required adjustments in PEEP to achieve a PL-exp within the range of 0–10 cm H2O. Notably, none of the patients had a PL-exp>10 cm H2O, nor was there a reduction in the initial PEEP setting. All 19 patients in the Constant PEEP group had an increase in PEEP, with the median of PEEP changes at 2 cm H2O [IQR 2–4].
Respiratory parameters showed variations between the groups. Airway DP displayed a significant variance, with medians of 15 cm H2O [IQR 13–16.8] in the Constant PEEP group and 19 cm H2O [IQR 16.2–20.9] in the Adjusted PEEP group (p=0.001). Similarly, respiratory system compliance (Crs) also demonstrated significant difference, with a median of 26.5 mL/cm H2O [IQR 24.3–29.2] in the Constant PEEP group compared to 20.6 mL/cm H2O [IQR 18.3–24.5] in the Adjusted PEEP group (p=0.001). Adjust PEEP level was +2 cm H2O [IQR 2–4] and the respiratory mechanics for the two groups are presented in Table 2.
Regarding the secondary outcomes, including mortality rate, duration of mechanical ventilation, and length of hospital stay, our analysis revealed no significant differences between the constant PEEP group and the adjusted PEEP group. Furthermore, our regression analysis, utilizing both univariable and multivariable logistic regression with a Backward stepwise approach, identified BMI, and initial PEEP settings as significant factors associated with adjusting PEEP to achieve a PL-exp above zero, as detailed in Table 3.
4. DISCUSSION
Critically ill patients frequently experience elevated chest wall weight and increased pleural pressures, secondary risk factors such as edema, pleural effusions, increased abdominal hypertension, and other causes [1,2]. These conditions contribute to alveolar derecruit-ment, increased lung elastance, and subsequent hypoxemia [1]. Prior investigations have underscored the efficacy of PEEP settings guided by esophageal pressure, targeting a positive PL-exp [4,5]. The Pes-guided PEEP group showed significantly better oxygenation with a 42% increase in PaO2/FIO2 and a 45% increase in respiratory system compliance at 72 hours. Due to the significant impact on oxygenation, this trial was terminated prematurely. Settings that achieve a transpulmonary pressure greater than zero were recommend-ed, especially in patients with a stiff chest wall or high pleural pressure [1,8]. Our study unveiled a 41.3% of incidence of positive PL-exp among ARDS patients (19 out of 46 patients). Moreover, our findings highlighted that BMI and initial PEEP settings played piv-otal roles in the adjusting PEEP to maintain positive PL-exp, We observed no significant disparities in secondary outcomes, including in-hospital mortality and length of hospital stay, between the constant and adjusted PEEP groups.
Certainly, ensuring PEEP levels to maintain a PL-exp greater than zero has been shown to mitigate atelectasis and the cyclical open-ing and closing of alveoli, improving pulmonary mechanics and oxygenation [9]. In our study, the initial PEEP set approach to the low PEEP-FiO2 strategy of the ARDSNet was associated with an increase in PEEP in 58.7% (27/46) of patients to achieve positive PL-exp. The EPVent trial conducted by Talmor et al. [5] which involved 61 ARDS patients, a Pes-guided PEEP titration strategy was compared with the low PEEP/FIO2 strategy. The findings revealed that 90% of patients in the Pes-guided PEEP strategy group required an increase in PEEP to achieve a transpulmonary end-expiratory pressure above zero. Similarly, Wang et al. [10] reported on 23 trau-matic ARDS patients where the esophageal pressure group had a mean of 12±4 cm H2O, higher than the PEEP titration value of 8±3 cm H2O in the ARDSNet group, with a significance of p<0.05. Additionally, the lack of notable variance in secondary outcomes, including in-hospital mortality and length of hospital stay between the two groups, underscores the safety associated with PEEP ad-justment. Therefore, it is suggested that in patients with moderate and severe ARDS, adhereing to the ARDSNet low PEEP/FiO2 table settings may still hold promise for lung recruitability.
Given the complexities of mechanical ventilation in high BMI patients, it is imperative to take into account the physiological changes. Bime et al. [11] highlighted that increased abdominal pressure and added mass of the chest wall in obese patients often ne-cessitate the use of higher PEEP levels to reduce the risk of atelectasis compared to non-obese. Similarly, Pirrone et al. [12] demonstrat-ed that the PEEP values employed in clinical practice (11±3 cm H2O) may be inadequate for optimazing ventilation in obese patients.
In Asian populations, where individuals typically have smaller anthropometric measurements compared ot those in European or American cohorts, the application of tritrated PEEP guided by esophageal pressure has been less explored. Additionally, it is notable that the World Health Organization has set a lower threshold for obesity in Asia-Pacific populations (BMI≥25 kg/m2) compared to the general standard (BMI 30≥kg/m2) [13–15]. In our study, the high BMI associated with increased PEEP to maintain positive PL-exp due to high BMI patients present with increased chest wall elastance and decreased pulmonary compliance, leading to lower or negative transpulmonary pressure values [16,17] Consistent with our findings, Mezidi et al. [18] reported that COVID-19 ARDS patients with a BMI>30 require higher PEEP (16 cm H2O versus 10 cm H2O) levels to achieve positive PL-exp. Furthermore, Kassis et al. also high-lighted the specific challenges in obese patients due to these alterations in chest wall elastance. They pointed out that the DP in such cases does not accurately reflect the true transpulmonary DP [17]. Therefore, we suggeste monitoring of transpulmonary pressure to titrate PEEP adjustments in patients with high BMI.
The research is subject to several limitations. Firstly, the single-center design and relatvely small sample size may limit the generali-zability of the findings to larger and more diverse populations. Additionally, the absence of intra-abdominal pressure measurements in our study is noteworthy. These limitations should be considered with caution when interpreting the results, emphasizing the need for more comprehensive future studies to build upon our findings.
5. CONCLUSION
The study showed that the incidence of positive PL-exp at 41.3% and adjusting PEEP may be beneficial in patients with high BMI in moderate to severe ARDS patients within Vietnamese populations. We suggest monitoring the transpulmonary pressure to individual-ize PEEP in high BMI patients. Further research is necessary to optimize and individualize PEEP settings in ARDS patients.
