1. INTRODUCTION
The progressive increase in the rate of cesarean sections (CS; C-sections) has led to rapid growth in the proportion of caesarean scar defects (CSD) and caesarean scar pregnancies (CSP) recently, creating an enormous burden for the healthcare systems in the world [1]. Similarly, CSP has become a significant challenge for Vietnamese healthcare system in recent decades. At Tu Du Hospital, the largest Obstetric and Gynecology hospital in the South of Vietnam, doctors have observed an upward trend in the number of CSP cases, from 287 cases in 2012 to 1,380 in 2019 [2].
Several contemporary studies have illustrated that a retroverted uterus, multiple cesarean scars, and single-layer closure of the uterus after a C-section are risk factors for CSD. Moreover, there is a relation between the volume of CSD and clinical symptoms. These studies have also shown that the clinical symptoms of CSD include post-menstrual bleeding, dysmenorrhea, chronic pelvic pain, and infertility [3–7]. However, there are no gold standard criteria exist for the diagnosis and treatment of CSD. Diagnosis is based on the patient’s main complaints and the results of medical imaging techniques. Depending on their functions, different tools may be employed, including 2D transvaginal sonography (TVS), 3D TVS, saline infusion sonohysterography (SIS), gel infusion sonohysterography (GIS), hysterosalpingography (HSG), and magnetic resonance imaging (MRI) [8–10]. Among these, ultrasound is the most commonly used method for managing CSD due to its convenience, cost-effectiveness and non-invasiveness.
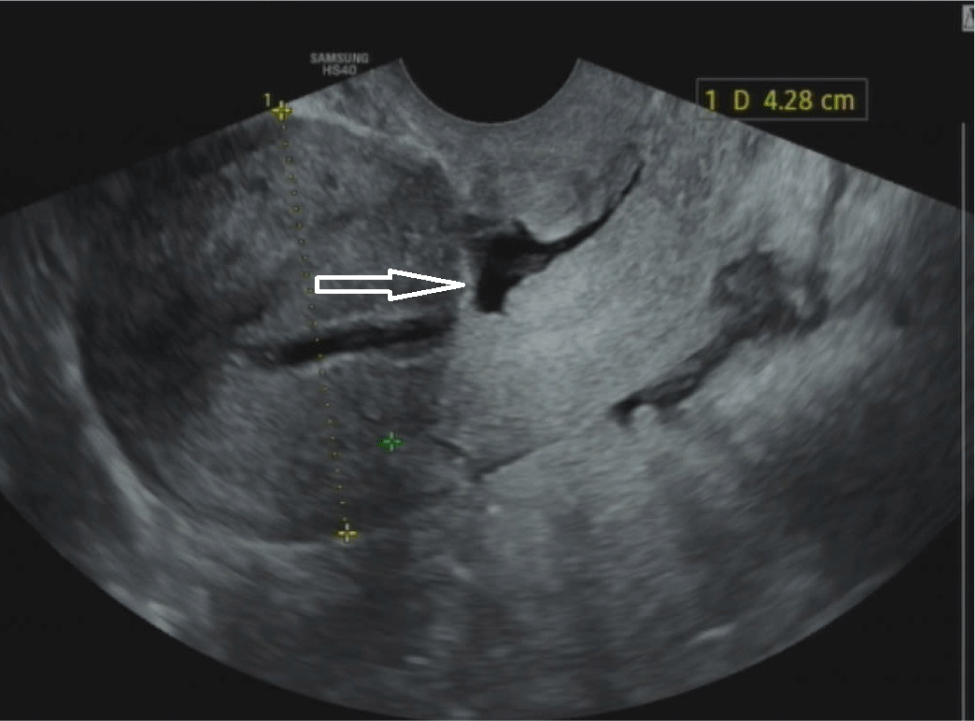
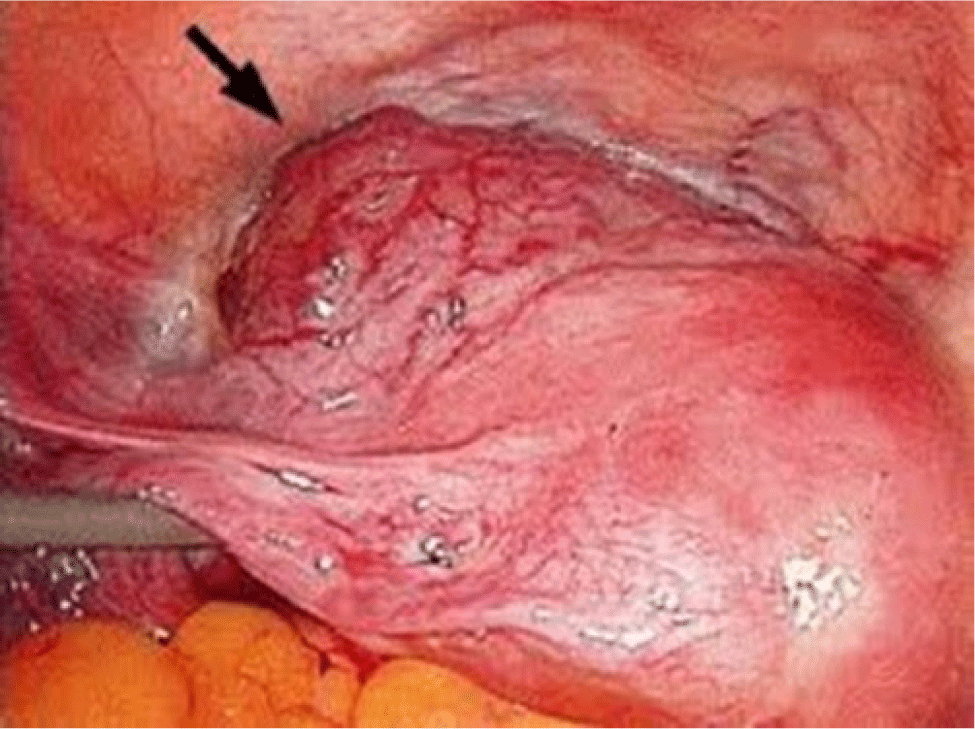
On the other hand, while there has been little experimental evidence to prove that CSD is the direct cause of CSP, a pathologic hypothesis has been proposed to highlight the invasion of a gestational sac in a niche of a previous C-section which creates complicated issues related to CSP in the first trimester and an adherent placenta in the third trimester. With the improvement of diagnosis and treatment as well as the development of sonography, the management of CSP has been improved in recent years (Figs. 1 and 2).
2. THE IMPORTANCE OF ULTRASOUND FOR A CAESAREAN SCAR DEFECTS (CSD)
A recent increase in studies about the significance of 3D, GIS, and SIS in the detection of CSD before surgery has been published [11,12]. Efforts have been made to explore the roles of 2D, 3D, and contrast-enhanced sonohysterography in assessing CSD.
The criteria of intervals between previous CS and CSD assessments are still unclear but should be at least 6 months after the previous C-section. Ideally, CSD should be evaluated 12 months post-surgery, as the healing process typically requires 3 to 6 months [1,11,12]. A study by Van der Voet et al. demonstrated that the residual myometrium thickness (RMT) of a CSD, assessed by ultrasound at 12-month after surgery, was significantly thinner compared to RMT of a CSD at 2-month point (p<0.05). Thus, there is a need to perform ultrasound at an adequate time point to effectively evaluate a CSD [13]. In addition, it is important to conduct the ultrasound assessment between 7th day to the 14th day of the menstrual cycle. During this period, the predominance of estrogen enhances contrast and minimizes the need of SIS and GIS. Moreover, this timeframe also provides an optimal opportunity to assess fluid retention, which could be a potential cause of infertility [12].
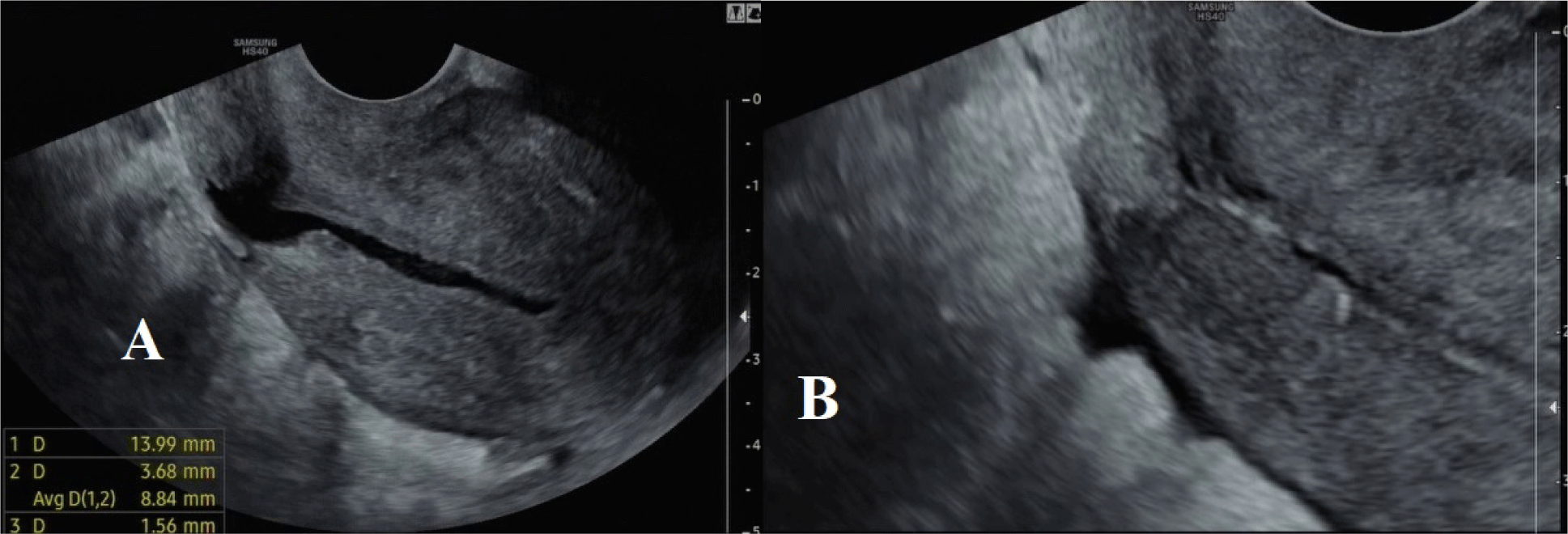
The standard sonographic criterion of CSD is the discontinuation of myometrium at the site of the previous C-section (a depth of at least 2 mm), which can be performed by 2D, 3D, SIS, or GIS ultrasound [10,12].
On the condition that there is a significant contrast of the endometrium, grey-scale TVS, also known as 2D TVS, is the fundamental imaging technique in screening for CSD. According to the results of a study conducted by Naji et al. (2012) and the following consensus of European experts in 2019, a guideline for evaluating CSD has been published [12,14].
On the mid-sagittal plane:
Residual myometrium thickness (RMT): measured from the apex of the CSD to the outer boundary of the myometrium, excluding fibrosis tissues. If a CSD is branched, it is necessary to evaluate the thinnest RMT.
Adjacent myometrium thickness (AMT): measured at the thickest adjacent myometrium. Length: measured at the base of a CSD, including the endometrium. Depth: measured from the base to the apex of the CSD.
Distance between niche and vesicovaginal fold: although it is optional, this measure provides more information about the boundary of CSD for surgeons before an operation. Distance between the CSD and the external os of the cervix: is the parallel line measured from the apex of CSD to the external os of the cervix.
Branching: The thinner part of the main branch tends to reach the uterine serosa at the site of CSD. Categories: simple CSD, simple CSD with one branch, and multi-branched CSD.
Size: A large CSD is defined when RMT is less than 2.2 mm on 2D TVS and/or less than 2.5 mm on SIS, GIS and/or a ratio of RMT/AMT is less than 0.5.
On the transverse plane:
Width: is the widest distance measured at the base of the CSD. If the CSD is branched, the longest width and length of every branch should be measured (Figs. 3 and 4).
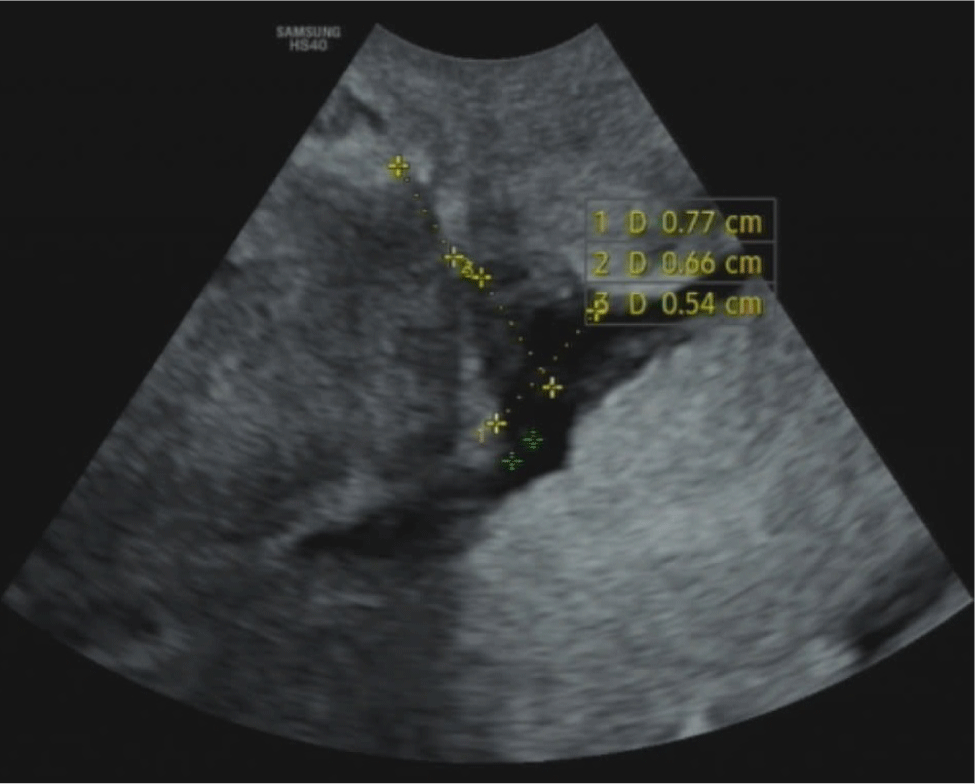
Evaluating the RMT and the RMT/AMT ratio plays a significant role in clinical practice. Bij de Vaate et al. mentioned a significant relationship between the volume of a CSD and postmenstrual bleeding, in which CSD’s average volume of the symptomatic group was higher than the figure of the asymptomatic group (p<0.05) [15]. Moreover, a large CSD is considered to be a high-risk factor in prospective pregnancies with severe complications such as uterine rupture and cesarean scar dehiscence. In a research conducted by Osser (2010), while 5.3% of the total patients with a small CSD (1/19) experienced those complications, the figure for the group with large CSDs was 42.9% (3/7) (p<0.05) [16]. The RMT and the AMT/RMT ratio are also crucial in selecting an appropriate surgical procedure. For treating CSD, operative hysteroscopy is typically remommended for patients with an RMT≥3 mm to reduce the risk of uterine perforation. On the other hand, operative laparoscopy is preferred for patients with an RMT<3 mm, as it helps increase the myometrium thickness, potentially decreasing the risk of uterine rupture in future pregnancies [10]. Due to the diversity in the shape of CSD, including triangles (83%), ovals (4%), circles (2%), and undetermined shapes without muscle layer (10%), measuring all the characteristics, including length, width, depth, branches and vesicovaginal folds, is essential for providing a comprehensive profile that aids surgeons in optimally repairing a CSD [4].
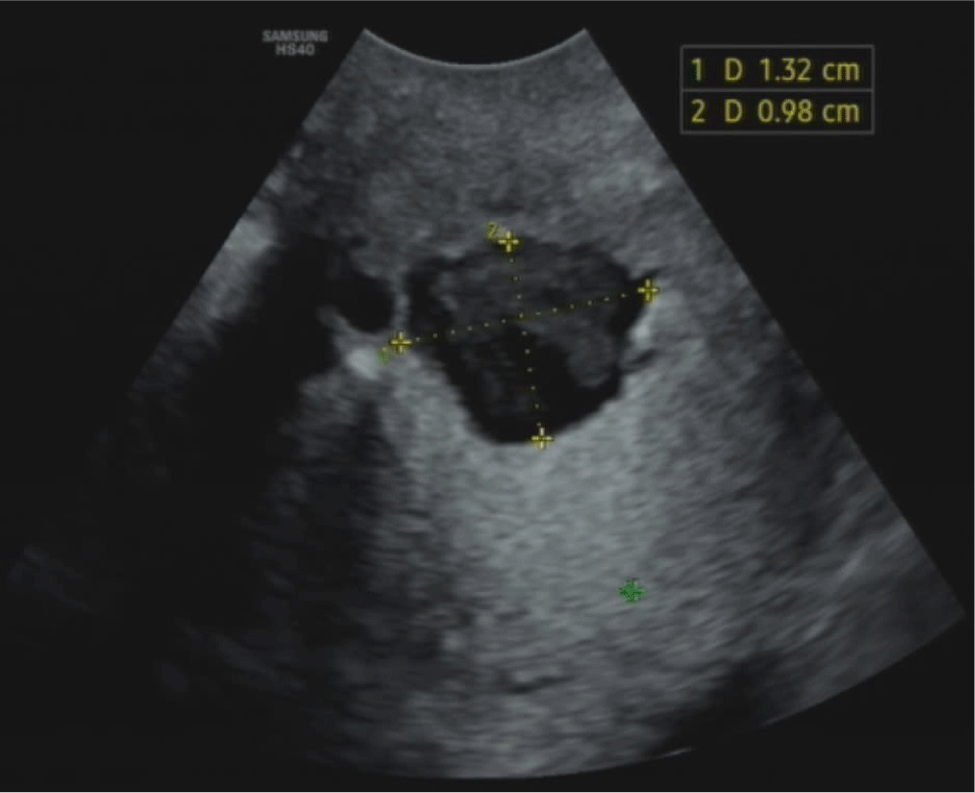
SIS and GIS are contrast-enhanced sonohysterographies that use saline or gel inserted into the uterus to provide clear view of the uterine lining on sonography (Fig. 5). The prevalence of detecting a CSD with SIS or GIS is 56%, which is twice as high as with grey scale ultrasound [15,16]. Moreover, SIS detection is 86% compared to a hysteroscopy (the gold standard) and evaluates a CSD more effectively than TVS [8]. Nevertheless, injecting saline or gel into the uterine cavity increases uterine pressure, which may exaggerate the actual size of the CSD. Additionally, SIS and GIS are not cost-effective, require more time, and can increase the risk of infection [14]. According to a consensus of European experts, using SIS or GIS is not justified if the grey-scale TVS can effectively identify a CSD. They assert that neither GIS and SIS offers a clear advantage over the other in such cases [12].
Recent technological advances have significantly enhanced the value of 3D TVS as a diagnostic tool, allowing clinicians to visualize the uterine cavity through virtual 3D simulations. 3D ultrasound can effectively render complex multi-branch structures of a CSD in the mid-sagittal plane [17]. However, despite its advanced capabilities, 3D TVS has shown a lower prevalence in detecting CSD compared to SIS and hysteroscopy, and its advantages remain inconclusive in recent studies [8]. Hence, further research is needed to establish standardized measurement criteria and to better define the role of 3D TVS in the management of CSD.
3. FUNCTIONS OF TVS IN CESAREAN SCAR PREGNANCY
TVS is commonly the first-lined method for diagnosing ectopic pregnancy, particularly in the managing CSP. Rotas et al. reported that TVS achieved an accuracy of up to 84.6% (CI 0.763–0.905) in diagnosing CSP [18]. The early detection of CSP enables clinicians to recommend appropriate treaments, thereby mitigating the risks of uterine rupture and severe hemorrhage, - two major complications that can significantly impact fertility.
Most CSPs are diagnosed in the first trimester based on the images of the mid-sagittal plane, showing the uterus to the gestational sac with high reliability. During an ultrasound, a heterogeneous echo often reveals the presence of a CSD. The RMT can typically be detected in the first-trimester images due to the progressive invasion of the CSP. In some challenging cases, evaluating the RMT may be difficult due to the gestational sac crossing over and causing serosal leakage.
Diagnostic criteria of a CSP have been agreed upon by many authors, including [19–22]:
-
- There is an empty uterine cavity without contact with the gestational sac.
-
- There is a clearly visible empty cervical canal without contact with the gestational sac.
-
- There is a discontinuity in the anterior uterine wall on the mid-sagittal plane running through the gestational sac.
-
- There is an image of a gestational sac with or without a fetal pole and with or without fetal cardiac activity (depending on the gestational age) in the anterior part of the uterine isthmus.
-
- There is a very thin or absent myometrium between the gestational sac and bladder.
Jurkovic et al illustrated a negative “sliding organ sign”, which indicates that the gestational sac cannot be displaced from its position at the cervical os by using gentle pressure with the endovaginal probe. This sign helps differentiate between a CSP and a spontaneous miscarriage in the cervix [23]. Nonetheless, some authors caution against this maneuver due to the risk of severe complications, such as life-threatening uterine bleeding, uterine rupture, or unnecessary surgery without proper resuscitation [24,25]. Jukovic et al. also suggested that transabdominal sonography (TAS) could provide clinicians with a comprehensive view of the uterus and pelvic organs, particularly the alignment between the uterus and bladder. They emphasized that performing TAS with a full bladder is especially valuable for assessing the distance between the gestational sac and the bladder [23].
Cali et al. introduced a new ultrasound marker called, “Crossover Sign” (COS) [26]. This marker assists physicians in evaluating the relationships between the gestational sac in the CSD and the front wall of the uterus to determine the development of the CSP. In the sagittal plane of the uterus, a straight line is drawn from the internal cervical os to the uterine fundus through the endometrium, referred to as endometrial line. The gestational sac is then identified and its superior-inferior (S–I) diameter, perpendicular to the endometrial line, is traced. COS is classified into two categories [27]:
-
- COS-1, the gestational sac is located in the Cesarean scar defect, and no less than two-thirds of the S–I diameter of the gestational sac is over the endometrial line, towards the front wall of the uterus.
-
- COS-2, the gestational sac is located on the Cesarean scar defect, and no more than two-thirds of the S–I diameter of the gestational sac is over the endometrial line. These cases are specifically divided into two distinct groups according to the presence (COS-2+) or absence (COS-2–) of an intersection between the S–I diameter of the gestational sac and the endometrial line (Fig. 6).
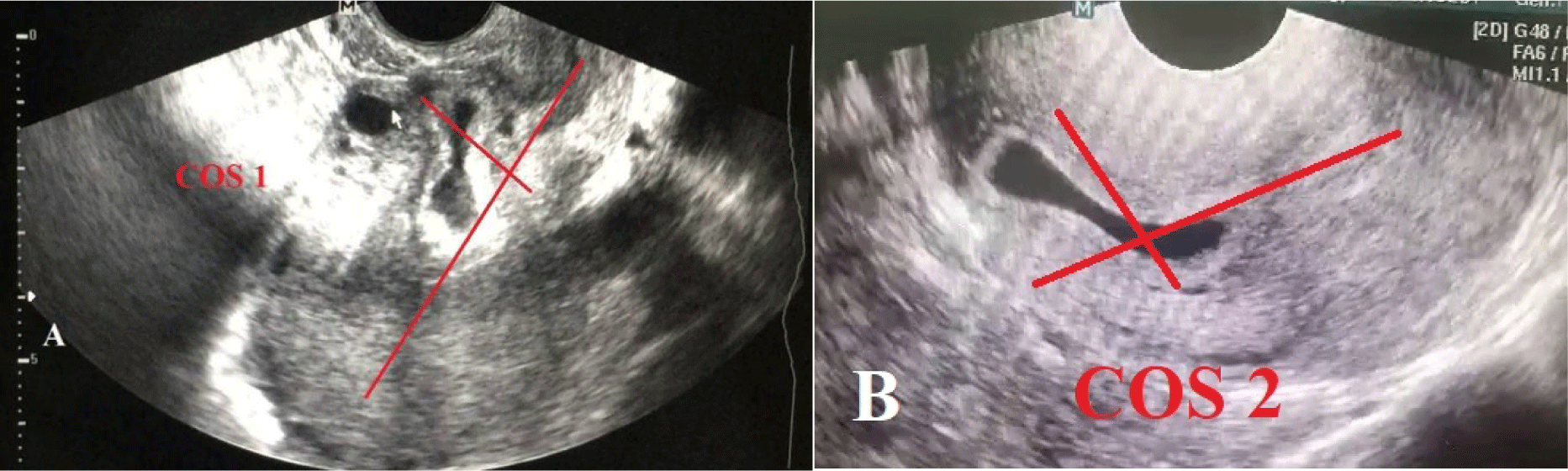
The potentially predictive value of COS for CSP treatment has recently been discussed, with COS1 identified as a detrimental factor. In 2021, Vo et al. conducted a case-controlled study on 161 CSP patients who had GA less than 8 weeks and were treated by the insertion of a foley catheter and intrauterine suction. The findings revealed that the proportion of patients with COS1 was 67.7% , the figure for COS2 was 32.3%. Among the COS2+ and COS2- accounted for 26.1% and 6.2%, respectively. In addition, the COS2 group had a significantly higher success rate with Foley catheter insertion followed by intrauterine suction after 24 hours compared to the COS1 group (OR=4.9, p<0.05). This group also experienced a shorter operative time (p<0.05) and reduced blood loss volume than the COS1 group (p<0.05) in cases the Foley catheter insertion and 24-hour intrauterine suction had previously failed [2]. This study builds on the findings by Cali et al. (2018), which indicated that patients with COS1 monitored from the 1st to the 3rd trimester were six times more likely to experience placenta accreta compared to those with COS2 (OR=6.67; p=0.001). Moreover, patients with placenta accreta experienced longer operation times, greater blood loss, and required more red blood cell units during a C-section compared to those without this condition (p<0.05) [27].
One of the benefits of Doppler ultrasound is to differentiate between CSP and spontaneous miscarriage at the cervix. In a miscarriage situation, Doppler ultrasound results typically show a flattened gestational sac with deformation in shape and compromised blood vessels. In contrast, CSP characterized by a high vascular flow index and low resistance index (RI), where high-velocity vascular flow and low RI around the embryo sac at the scar remain relatively stable during the first trimester [18]. The detection rich peritrophoblastic blood flow (Vmax>20 cm/s, RI<1) plays a significant role in predicting CSP treatment. Compared to patients without vascular proliferation, Vo et al. (2021) mentioned that the likelihood of treatment failure was 24.75 times higher among ones with rich vascularization (p<0.05). The absence of vascular proliferation on Doppler ultrasound could indicate a regressive CSP. In contrast, high vascular proliferation, which signifies the development of the gestational sac, increases the risk of bleeding and can lead to treatment failure. Similarly, the probability of treatment failure is 3.78 times higher among patients with a CSP volume>4 cm3 [2].
4. LIMITATION AND FUTURE RESEARCH
This review article presents limited data from randomized control trials (RCTs). As such, there is a need for prospective RCTs to be conducted in order to gather more evidence regarding the role of sonographic imaging techniques in the management of CSD and CSP.
5. CONCLUSION
Transvaginal sonography plays a crucial role in the management of both CSD and CSP. Based on appropriately measuring RMT, AMT, RMT/AMT ratio, depth, width, and length of CSD and categorizing CSD, clinicians can perform efficient treatments for patients to have better health outcomes. Besides that, COS, vascular proliferation, and volume of a gestational sac through Doppler ultrasound are considered to be essential signs in the management of CSP. For this reason, transvaginal ultrasound with suitable measurement should be utilized widely in clinical practice.