1. INTRODUCTION
Dyslipidemia, also referred to as hyperlipidemia or hypercholesterolemia, is the primary risk factor for high blood pressure, myocardial infarction, stroke, and atherosclerosis [1,2]. Therefore, lipid tests are routinely performed in the laboratory for early diagnosis of lipid disorders and prevention of cardiovascular diseases [3,4]. Previous studies have shown that sample collection, storage, and transport are pre-analytical factors that can influence the accuracy of laboratory testing [5–7]. The literature provides different opinions on the stability of lipids [8,9]. Some studies have recommended that laboratories freeze plasma or serum samples as soon as possible to ensure sample stability [8], while the MONICA Manual recommends the determination of high-density lipoprotein-cholesterol (HDL-C) and total cholesterol (TC) on fresh serum aliquots on the day of blood collection [9]. TC, HDL-C and triglyceride (TG) concentrations can be determined in serum or in plasma samples separated from anticoagulated whole blood (WB) samples [10]. However, there are many recommendations to use plasma instead of serum in lipid studies by drawing blood in the presence of ethylenediaminetetraacetic acid (EDTA), heparin or other anticoagulants [11,12]. Significant differences in lipidomic profiles between plasma and serum have been demonstrated [11]. According to World Health Organization (WHO) guidelines, plasma samples containing anticoagulants such as lithium-heparin, EDTA, or citrate can be used to determine TG, TC, and HDL-C concentrations without affecting the results [13]. Some previous studies evaluated the stability of lipids in plasma samples containing the anticoagulant lithium-heparin or EDTA [8,14]. EDTA is an anticoagulant that can be used on blood samples to analyze blood cell morphology, while lithium-heparine is limited. When necessary, the remaining EDTA anticoagulated WB sample can be used to separate plasma for TC, HDL-C, and TG quantification. Moreover, many clinical studies are performed on samples stored for weeks or months until analysis. However, sampling techniques, storage temperatures, and analytical procedures can lead to degradation of analyte compounds in the sample [11]. Currently, studies on the stability of TGs, TC, and HDL-C under different storage conditions are still limited in Vietnam. The objective of this study is to evaluate the stability of TC, HDL-C, and TGs in human WB and plasma samples under different storage conditions to contribute to improving the accuracy of test results.
2. MATERIALS AND METHODS
The concentrations of HDL-C, TC, and TGs were determined on biochemical autoanalyzer Erba XL640 (Erba Mannheim®, Miami, FL, USA), which met in vitro diagnostic (IVD) certificate and were checked with internal control samples for TC, TGs, and HDL-C each day before quantification of TC, TGs, and HDL-C in the blood samples. Commercial biochemical kits used for quantification of HDL-C, TC, and TG concentrations were purchased from Erba Lachema s.r.o. The internal quality control panel for biochemical testing with 2 levels (normal and high) was purchased from Randox® (Crumlin, UK).
Blood sample collection for this study was approved by the Ethics Committee of University of Medicine and Pharmacy at Ho Chi Minh City and issued together with Decision No. 759/ĐHYD-HĐĐĐ. Blood donors have met the criteria for blood donation according to Circular 26/2013/TT-BYT issued on September 16, 2013 such as adults from 18 to 60 years old, with a minimum weight of 42 kg for women and 45 kg for men, and do not use any drugs.
Sample size determination for this study was referenced from sample size summary reports of previous studies [15]. The fasting venous blood sampling from volunteers were performed according to CLSI guidelines [16] and previous studies [12,14].
Ten volunteers (five men and five women) consented to povide overnight fasting whole venous blood into a vacuum blood collection. Each provided of 10 mL of WB into a vacumme blood collection tubes containing dipotassium dipotassium ethylenediaminetetraacetic acid (EDTA [K2-EDTA]. Of this, 5 mL of WB was used to separate the plasma fraction through centrifugation, while the remaining 5 mL was left untreated. Plasma samples were separated from WB by centrifugation within 30 minutes of collection at room temperature (20°C–25°C) [17]. Exclusion criteria for blood samples included missing sampling information, clotted blood, hemolyzed blood, cracked or broken sample tubes, and the presence of anticoagulants other than K2-EDTA.
WB and plasma samples from each volunteer were aliquoted into sterile tubes (0.5 mL each) to assess lipid stability under different temperatures conditions over time. Plastic test tubes used for blood or plasma samples were sterile, and stable at the investigated temperatures, ensuring no reaction with the analytes.
All procedures for blood sampling, sample processing, and quantification of TC, TGs, and HDL-C were carried out at Buon Ma Thuot Medical Testing Center, which has been accredited to meet International Organization for Standardization (ISO) 15189:2012 standards for laboratory biosafety cabinet - Class II.
Most biochemical tests are generally recommended to be performed at room temperature. Moreover, the stability of biochemical parameters is generally higher when samples are stored at refrigerator temperature [18,19]. The intervals for evaluating changes in TC, TG, and HDL-C concentrations in this study were determined based on previous research results and recommendations regarding the stability of TC, TGs, and HDL-C in both plasma and WB samples [12–14].
WB and plasma samples for this study were stored at temperatures of 20°C–25°C and 2°C–8°C. All WB samples were processed by centrifugation to separate the plasma fraction before determining TC, TG, and HDL-C concentrations by spectrophotometry.
At 20°C–25°C, TC, TG, and HDL-C levels in WB and plasma samples were measured at times points 0 (T0), 4 (T4h), 12 (T12h), and 24 hours (T24h). TC, TGs, and HDL-C levels in each WB and plasma samples from 10 volunteers were measured induplicate.
At 2°C–8°C, TC, TG, and HDL-C levels in WB and plasma samples were measured on days 1 (T1d) and 2 (T2d). WB and plasma samples stored at 2°C–8°C should be conditioned at room temperature for 15 minutes before quantification and should not be reused for subsequent testing.
The concentrations of TGs, TC, and HDL-C in WB (WB-TGs, WB-TC, WB-HDL-C, respectively) and in plasma samples (P-TGs, P-TC, P-HDL-C, respectively) were determined simultaneously on biochemical autoanalyzer XL 640 using enzymatic spectrophotometry. Lipid quantification procedures were performed according to the manufacturer’s instructions. The absorbance measured at a wavelength of 550 nm is proportional to the lipid concentration in each of the reactions.
The stability of TC, TGs, and HDL-C in WB and plasma samples was assessed by comparing TC, TGs, and HDL-C concentrations at the following time points with baseline concentrations (T0). If the concentrations of TC, TGs, and HDL-C became unstable at any tome point, subsequent measurement of TC, TGs, and HDL-C concentrations would not be necessary. The influence of the sample matrix on TC, TGs, and HDL-C was investigated by comparing the sdifference between TC, TGs, and HDL-C concentrations in WB and plasma samples at each testing time.
3. RESULTS
The baseline concentrations of TC, TGs, and HDL-C in the human plasma samples determined (T0) at room temperature on Erba XL640 Analyser were in the range of 4.74±0.75, 2.48±1.31 and 1.68±0.28 mmol/L, respectively (Table 1).
The results showed that the average concentrations of TG and TC in WB samples (WB-TGs, WB-TC) and in plasma samples (P-TGs and P-TC) at 4 hours were significantly different from the baseline concentrations of TC and TG (p<0.0001), while HDL-C concentrations in WB and plasma samples (WB-HDL-C and P-HDL-C, respectively) were stable over 4 hours compared to the baseline concentration of HDL-C (HDL-CT0) (p>0.05) (Tables 1 and 2). Therefore, the concentrations of TG and TC in WB and plasma samples could not be determined at 12 hours. Since WB-HDL-C and P-HDL-C concentrations at 12 hours were significantly different from HDL-CT0 levels (p<0.05), these concentrations were not determined at 24 hours (Tables 1 and 2).
At 20°C–25°C, there were no significant differences in TG, TC, and HDL-C concentrations in WB samples compared to plasma samples at the 4-hour measurement time points after sampling. However, only HDL-C concentrations in WB and plasma samples were not significantly different at 12 hours after sampling (Fig. 1).
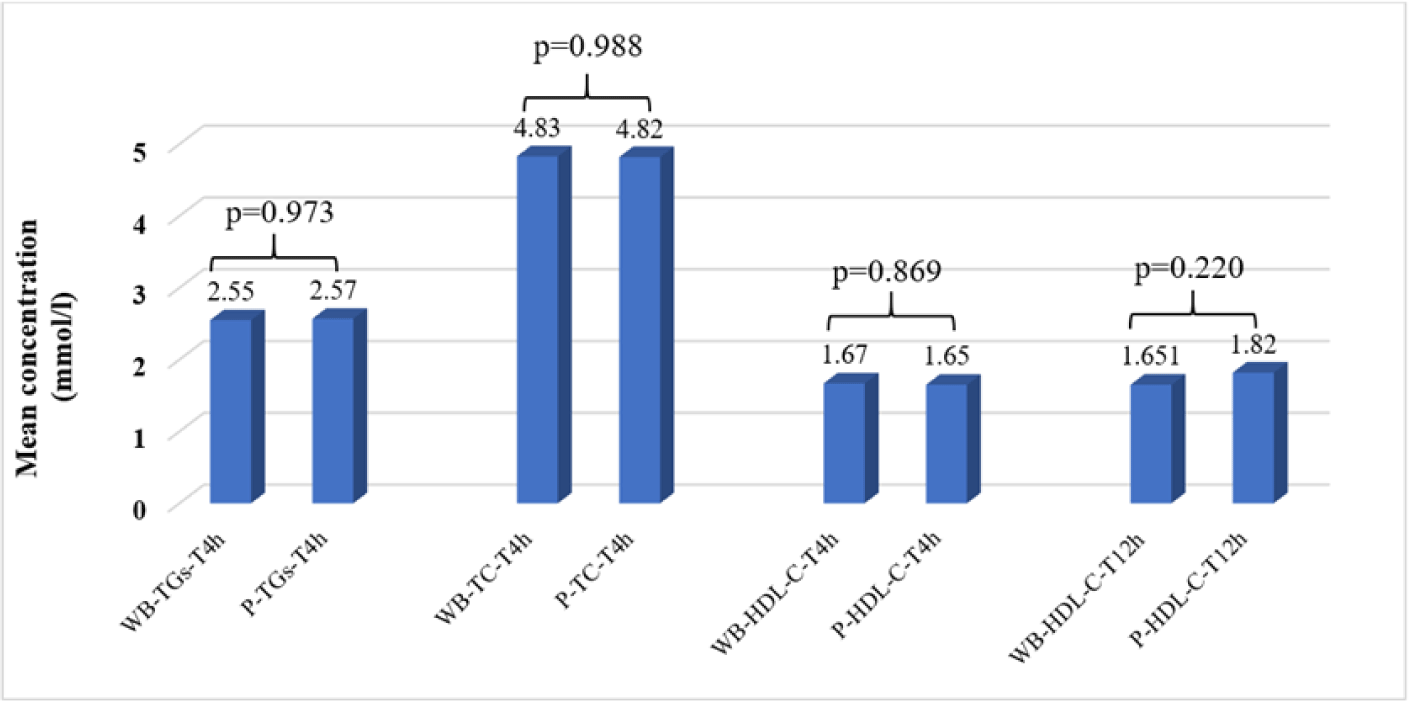
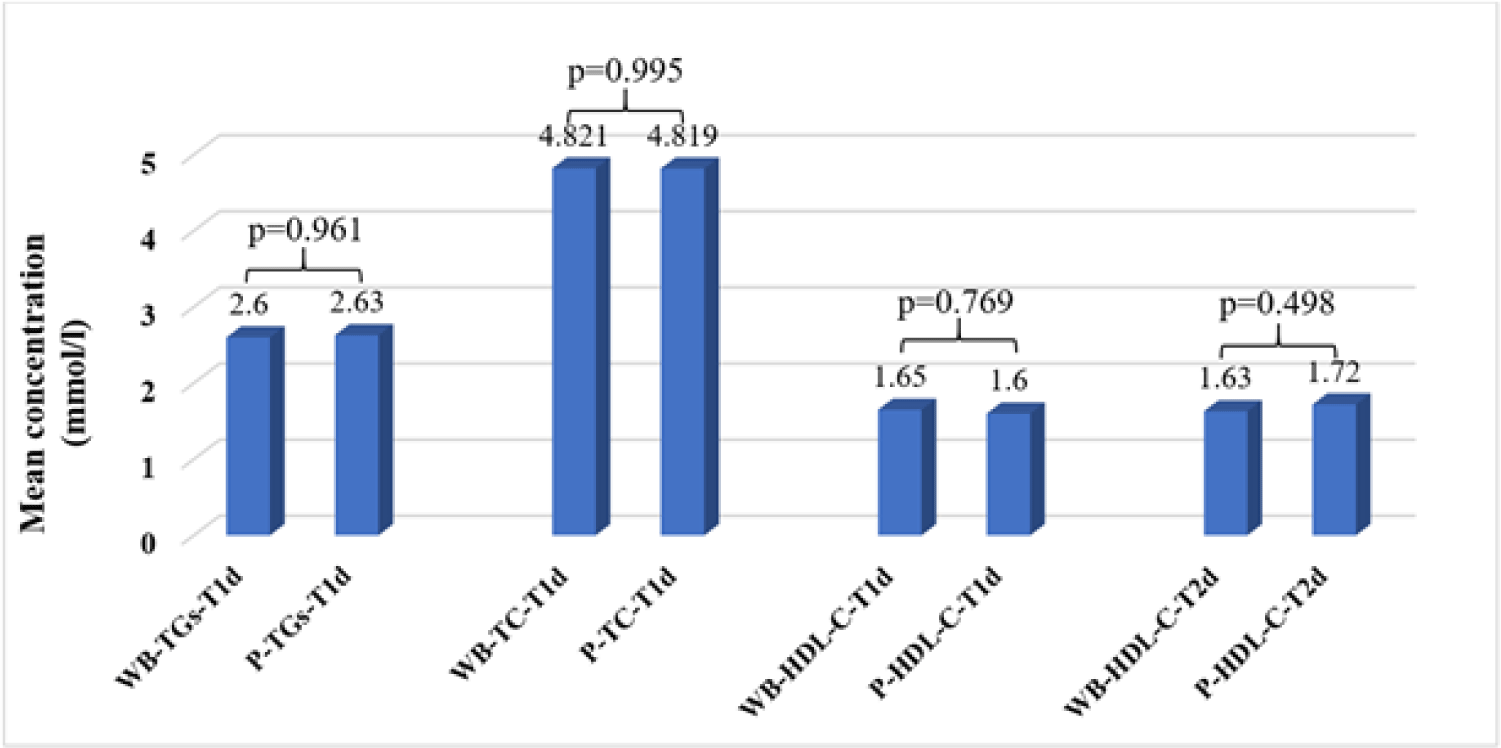
The average concentrations of TGs, TC, and HDL-C in WB samples stored at 2°C–8°C were not significantly different with the baseline on TCT0, TGsT0, and HDL-CT0, respectively (p<0.05), but were stable for 48 hours (p<0.05) (Table 3).
Since the average concentrations of TGs and TC in plasma samples stored at 2°C–8°C were only stable for less than 24 hours compared to TCT0, TGsT0 (p<0.05), the P-TGs and P-TC concentrations were no longer determined at T2d (Table 4). The average plasma concentrations of HDL-C at 2°C–8°C were stable under 48 hours (p<0.05) (Table 4).
At 2°C–8°C, there were no significant differences in TG, TC, and HDL-C concentrations in WB samples compared to plasma samples at the 24-hour measurement time points after sampling. However, only HDL-C concentrations in WB and plasma samples were not significantly different at 48 hours after sampling (Fig. 2).
4. DISCUSSION
Sample stability is very important to obtain reliable concentrations of many analytes, including lipids [20]. Differences in sample processing times between studies may be due to sample collection time, laboratory conditions, and sample quantity. In fact, some clinical laboratories take too many samples and cannot process them promptly, or due to the need for many patients to receive medical care right at home sample collection can take longer than the time prescribed by the manufacturer’s recommendations [5–9]. TC, HDL-C, and TG concentrations can be determined in serum or in plasma samples separated from WB samples anticoagulated with various anticoagulants [11,12]. EDTA is an anticoagulant that can be used on blood samples to analyze blood cell morphology, while lithium-heparine is limited. When necessary, the remaining EDTA anticoagulated WB sample can be used to separate plasma for TC, HDL-C, and TG quantification. Moreover, many clinical studies are performed on samples stored for weeks or months until analysis [11,12]. Our study evaluated the stability of HDL-C, TC, and TGs over time at different temperatures using Erba XL640 Analyser. This automatic biochemical analyzer has been commonly used in small and medium-sized laboratories in urban areas, so the results of this study can be a reference value for laboratories using similar analyzer systems.
Many laboratories in Vietnam currently recommend that specimens should be analyzed as soon as possible. Remaining serum or plasma samples separated from EDTA-anticoagulated WB samples used for biochemical and immunological testing can be stored and stabilized at room temperature (≤25°C) within 48 hours or at 2°C–8°C within 7 days. These samples can be preserved for a long time if frozen at temperatures below –20°C [18,21]. Our study compared the stability of TC, TGs, and HDL-C in plasma and WB samples at the same storage conditions. Therefore, WB samples cannot be stored at –20°C. Furthermore, multicenter studies encounter difficulties related to centrifuging and freezing samples immediately after blood collection. For that reason, the stability of TGs, TC, and HDL-C in WB and plasma samples stored at 20°C–25°C and 2°C–8°C was evaluated in this study. The results of our study showed that the stability of plasma TGs, TC, and HDL-C stored at 20°C–25°C and 2°C–8°C was lower than the recommended conditions [18,21].
Previous study has shown that processing time and storage conditions did not affect TC, TG, and HDL-C concentrations measured after 2–3 hours at 8°C and 4–5 hours at room temperature from collection [19]. In our study, the concentrations of plasma TGs and TC were stable for less than 4 hours at 20°C–25°C and 24 hours at 2°C–8°C, while the concentrations of plasma HDL-C were stable for less than 12 hours at 20°C–25°C and 48 hours 2°C–8°C. The results of our study showed that the stability of TGs, TC, and HDL-C in plasma samples was not the same at different temperatures.
The assessment of TG, TC, and HDL-C stability in WB has not been consistent between studies. TC, HDL-C, and TG concentrations in WB stored at room temperature for up to 7 days have been shown to vary by less than 4% [12]. However, another study found that all blood samples were completely hemolyzed after being stored for seven days. In our study, the concentrations of TGs and TC in WB samples were stable for less than 4 hours, while the concentrations of WB-HDL-C were stable for less than 12 hours at 20°C–25°C. At 2°C–8°C, the concentrations of WB-TGs, WB-TC, WB-HDL-C, and P-HDL-C were stable up to 48 hours. Therefore, HDL-C concentrations are considered more stable than TG and TC in both plasma and blood samples.
There was a slight increase in mean plasma concentrations of TGs, TC, and HDL-C at different temperatures over time compared to baseline levels (T0). These results were similar to previous studies [20,22,23], although the analysis methods and sample analysis systems were different. However, other studies showed that TGs and HDL-C concentrations in human WB were significantly different after 10 hours of storage at 21°C [24], while plasma TC tended to increase after 56 hours of storage at 25°C [17]. The cause of increased plasma lipids during storage is unknown [25]. The analysis techniques, temperature and sample storage time could lead to differences in research results.
Previous studies have shown that some biological analytes changed significantly when blood samples were stored for up to 24 hours at temperatures above room temperature [20,25]. Therefore, biological samples should be stored properly and transported to the laboratory for a short analysis period. In this study, the influence of the sample matrix on TC, TGs, and HDL-C was investigated by comparing the difference between TC, TGs, and HDL-C concentrations in WB and plasma samples at each testing time. This study found that there was no significant difference in the stability of TGs, TC, and HDL-C in WB and plasma samples under the same storage conditions and determined at the same testing time. However, if it is not possible to quantify TC, TGs, and HDL-C concentrations immediately or if it is necessary to save samples for re-tests, WB samples could be stored at 2°C–8°C for 48 hours for the measurement of concentrations of TC, TGs, and HDL-C.
This study only investigated the stability of TG, TC, and HDL-C in blood samples collected from fasting healthy volunteers under different temperature conditions. These volunteers were not sick or taking any medications at the time of sample collection. A limitation of the study is that factors affecting the stability of lipid parameters have not been evaluated. Further studies could evaluate the influence of storage conditions on the stability of TG, TC, and HDL-C in non-fasting blood samples, and other factors related to stability of blood lipids such as fasting time and, comorbidities.
5. CONCLUSION
This study evaluated the stability of TC, TGs, and HDL-C under the influence of storage conditions and biological matrix of samples. The stability of TGs, TC, and HDL-C in WB and plasma samples was not completely the same at different temperatures. The remaining EDTA-anticoagulated WB samples stored for 2 days at 2°C–8°C can be used for determination of TC, HDL-C, and TGs. In future studies, the effect of storage conditions on the stability of normal and abnormal levels of TC, TGs, and HDL-C may continue to be evaluated.
