1. CASE REPORT
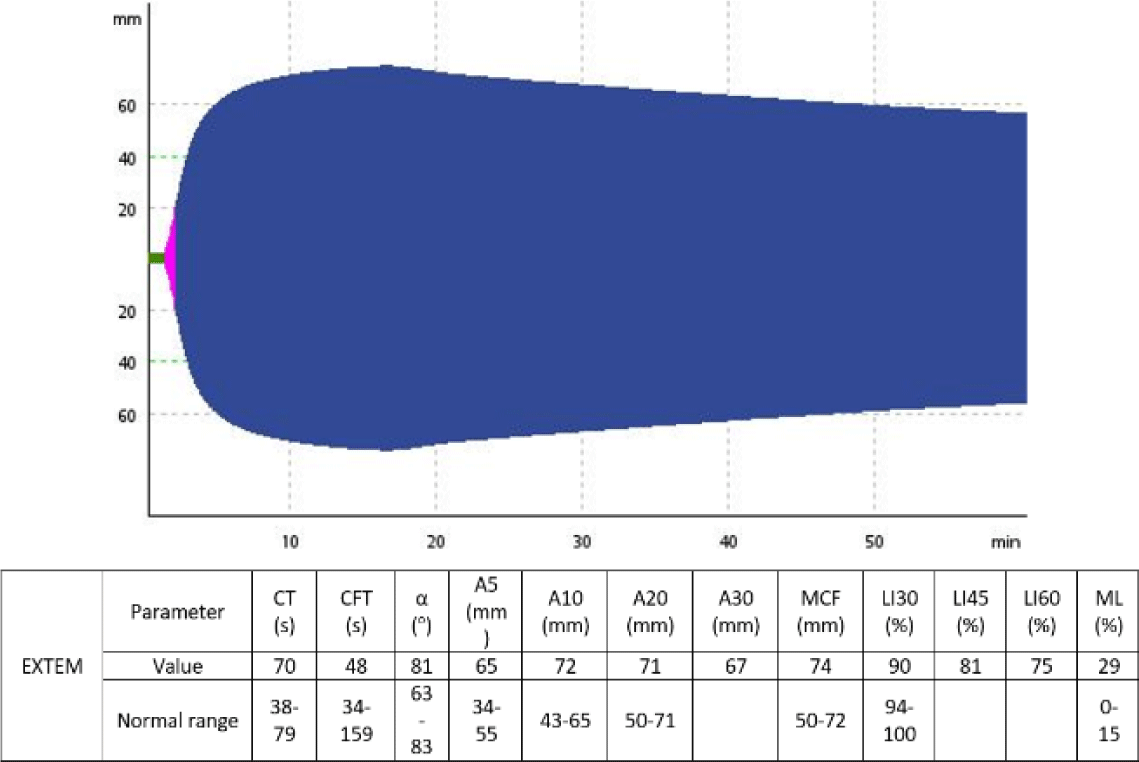
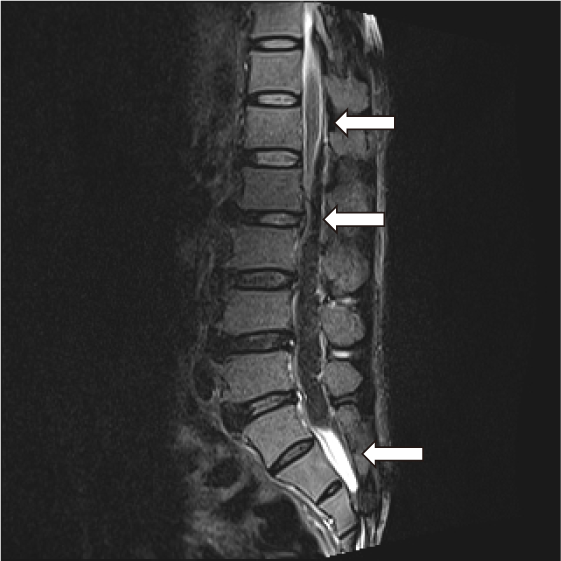
A 62-year-old man was admitted to the Emergency Department due to back pain and gradual paraplegia after bending over to clear a clogged drain. Upon admission, loss of sensation and voluntary movement was detected in his lower body. Routine laboratory assays revealed an increased hematocrit of 69.6%, an increased platelet count of 750×10e9/L, an increased white blood cell count of 40.49×10e9/L and normal coagulation parameters including prothrombin time, activated partial thromboplastin time and fibrinogen level. Magnetic resonance imaging (MRI) of the spine showed epidural hemorrhage spreading from L1 to S1 epidural space, causing severe lumbar spinal stenosis (Fig. 1). Given that the patient’s bleeding was clearly not traumatic-induced and routine coagulation results was unremarkable, PFA-100 (Dade Behring, Newark, DE, USA) assays, von Willebrand factor antigen and activity quantification and a set of ROTEM (Tem Innovations GmbH, Munich, Germany) tests including INTEM, EXTEM and FibTEM were ordered to rule out any possible hemostatic disorder. The ROTEM assays revealed markedly hyperfibrinolysis, with decreased LI30, LI60 and increased ML observed in all ROTEM assays. Other parameters were within normal ranges.
Furthermore, von Willebrand factor activity/antigen ratio was >0.7 and PFA-100 Collagen-Epinephrine (CEPI) and Collagen-Adenosine diphosphate (CADP) closure times (CT) were prolonged at 300 (normal range 84–160 seconds) and 253 seconds (normal range 68–121 seconds), respectively, suggestive of platelet dysfunction. As a result, he was given tranexamic acid at 500 mg every 8 hours and platelet transfusion. His plasminogen level and activities of factor XIII, α2-antiplasmin and plasminogen activator inhibitor-1 (PAI-1) were also checked to clarify the mechanisms behind his hyperfibrinolytic condition. PAI-1 activity was undetectable, while the other test results were within normal ranges. Bone marrow biopsy and JAK2V617F mutation detection via allele-specific polymerase chain reaction (PCR) were performed. The diagnosis of polycythemia vera (PV) was confirmed as bone marrow biopsy was hypercellular for the patient’s age, and showed trilineage hematopoiesis, including erythroid, granulocytic, and megakaryocytic proliferation with pleomorphic, mature megakaryocytes, and JAK2V617F mutation was detected. Consequently, treatment included phlebotomy and cytoreduction with hydroxyurea (1.5 g/day) was indicated to lower his hematocrit and platelet count.
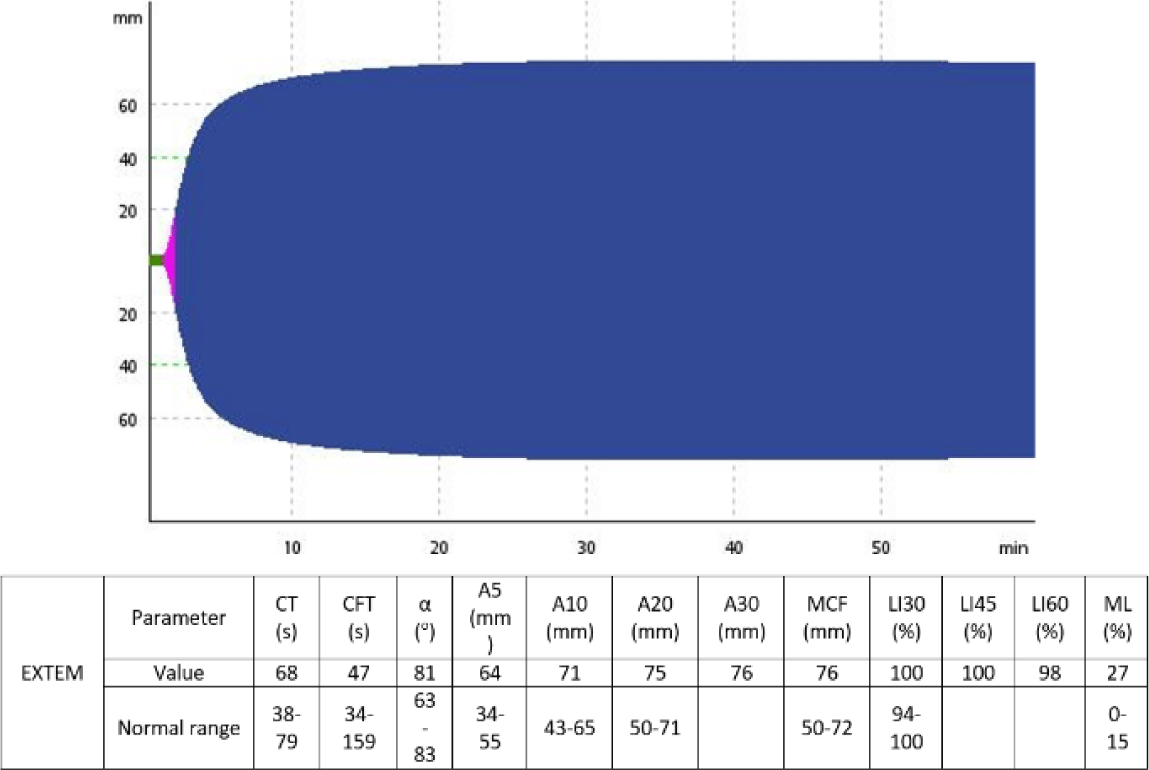
After 3 tranexamic acid doses, a second set of ROTEM and PFA-100 assays were performed to monitor his hemostatic status and showed improvements in lysis indexes such as LI30, LI60, as well as increased PAI-1 level to 4.86 unit/milliliters (normal range 0.3–3.5 unit/milliliters) and decreased CEPI and CADP closure time. The patient required decompressive laminectomy to manage his hematoma, with tranexamic acid maintained throughout the perioperative period at increased doses of 500 mg every 4 hours. No excessive bleeding was recorded during and after the surgery. The patient fully recovered from his paraplegia and was discharged on oral tranexamic acid at 500 mg every 8 hours after 3 weeks of hospitalization. Fig. 2 and Fig. 3 show the change of the patient’s EXTEM results before and after the administration of tranexamic acid.
2. DISCUSSION
Bleeding is uncommon in PV, with only 3%–8.1% of patients presenting with this symptom at the time of diagnosis [1]. However, hemorrhage can be severe in this patient population, as evidenced by our case. There are several factors contributing to the bleeding tendency of PV patients, most notably platelet quantitative and qualitative disorders and acquired von Willebrand syndrome [1,2]. In our case, we excluded acquired von Willebrand syndrome, as the patient’s von Willebrand factor activity/antigen was >0.7 [3]. Platelet dysfunction in PV has been described in multiple reports as reduced aggregation to epinephrine, ADP and collagen [4]. PFA-100, an optical-based platelet function analyzer, has been used to detect platelet qualitative defects in primary thrombocythemia, another myeloproliferative neoplasm (MPN) [5]. This system also helped us to identify our patient’s platelet dysfunction. However, the link between platelet function and bleeding risk in PV and other MPNs is still controversial, since there are reports showing that platelets in MPNs are hyper reactive, resulting in a paradox of thrombosis and bleeding [4]. Moreover, in vitro platelet defects have been observed in all MPN patients, regardless of their bleeding and thrombotic statuses, further highlighting the uncertainty in the relationship between bleeding diathesis and platelet function disorders in MPNs [4]. It is thus likely that platelet defects can only lead to significant bleeding events when occurring simultaneously with other pro-hemorrhagic disorders.
Hyperfibrinolysis is a well-known mechanism of excessive bleeding, particularly in trauma patients [6]. In PV, however, there are very few reports on its incidence and role as a bleeding risk. In one such study, PV patients have been shown to have decreased α2-antiplasmin and plasminogen levels, suggesting of a hyperfibrinolytic state [7]. Our patient’s α2-antiplasmin and plasminogen levels were within normal ranges, but his PAI-1 activity was undetectable at presentation. PAI-1 deficiency is usually congenital, and is linked with late-onset hemorrhages [8]. Nonetheless, in our case, the patient’s PAI-1 activity bounced back after treatment, suggesting a consumptive mechanism behind his deficiency, similar to one seen in trauma patients with disseminated intravascular coagulation [6]. In general, the incidence, mechanism and importance of hyperfibrinolysis in PV remain obscure, and future studies need to be done to enlighten these issues.
One potential obstacle for research on bleeding risk factors in PV is the lack of reliable assays to comprehensively analyze the hemostatic process. ROTEM, a global coagulation assay, can reliably assess almost all the clot formation steps, including fibrinolysis [9] (EXTEM ML>15% is a common definition for hyperfibrinolysis in clinical studies [9]) However, standard ROTEM tests are insensitive to platelet aggregation inhibition [10] and reduced von Willebrand factor activity [11]. Therefore, we can only obtain an adequate picture of a patient’s coagulation process when we add a platelet aggregation assay like PFA-100 and von Willebrand factor activity and antigen quantification to the standard ROTEM panel.
To treat hemorrhages in PV, current guideline suggests the use of tranexamic acid [12]. Tranexamic acid is also recommended for treating patients with hyperfibrinolysis, as it blocks the interaction between plasminogen and fibrin, and directly inhibits fibrin degradation [13]. However, tranexamic acid is also associated with an increased risk of thrombosis [14], which may complicate the situation as PV is already a prothrombotic condition [1]. Furthermore, another aspect of bleeding management in PV patients is optimal cytoreduction [12], as thrombocytosis and leukocytosis have been shown to be linked to hemorrhage risk [12], but some patients, like ours, may contradictorily require platelet transfusions for bleeding control [12]. Considering the complexity of bleeding management in PV, especially in cases with hyperfibrinolysis, we believe that close monitoring with specific coagulation assays should be performed to ensure patients’ safety and successfully individualizing treatment.
3. CONCLUSION
Hemostatic complications are the leading cause for mortality in MPNs like PV. While hemorrhagic events are not frequently encountered in PV [1], they may lead to catastrophic consequences if not appropriately managed. However, bleeding control in this condition is complex, as multiple intertwined mechanisms contributing to the patients’ bleeding diathesis [1]. Hyperfibrinolysis, despite its well-known status as a leading cause for hemorrhages in the general population [2], is not studied thoroughly in PV. Therefore, we believe that more carefully-designed research is needed to address this issue. For the time being, we suggest that every bleeding PV patient should be evaluated in a holistic approach using specific assays such as ROTEM or PFA-100 to tailor his/her treatment to his/her particular etiologies.