1. INTRODUCTION
Tablets are one of the most commonly used dosage forms of medication due to its convenience and cost-effectiveness. However, the manufacturing process for the tablet involves multiple stages and various technologies (e.g., granulation, drying, and tableting technologies). Among those, the tableting stage, where the powder or granule blend is compressed into tablets, involves complex phenomena and kinetics. At this stage, out-of-control factors such as blend compositions or process parameters may occur, potentially resulting in tablet quality defects (i.e., punch sticking, tablet capping, chipping, mottling, etc.) and even reducing the efficiency and yield of the manufacturing process [1].
Punch sticking is a typical tablet defect that occurs when the adhesion force between the particles in the final powder blend and the punch surface exceeds the cohesive force between the particles in the blends [2]. Various factors can induce this phenomenon, including process parameters and material properties. Physicochemical factors (e.g., the morphism [3], particle size [4], and melting point [5]) of the active pharmaceutical ingredient (API) , as well as the type and concentration of fillers [6] or lubricants, [7] have been shown to impact the punch-sticking propensity. An increase in temperature during compression can also contribute to this kind of defect [8]. To effectively design strategies to mitigate the risk of punch sticking, it is essential to achieve a mechanistic understanding of the process parameters and material properties that can affect sticking propensity.
Although several studies have been published, experimental data on the influence of APIs properties and formulation composition on the risk of punch sticking remain incomplete. The aim of this study was to investigate the influence of formulation components and punch properties on sticking and to provide data supporting a better understanding of strategies to mitigate this defect. The factors to be explored included model API properties (e.g., particle mean size, melting point), formulation composition (including drug loading, type of fillers, type of lubricants), as well as the material and geometry of punch. The data obtained may serve as a reference for the formulators when addressing the issue of punch sticking.
2. MATERIAL AND METHOD
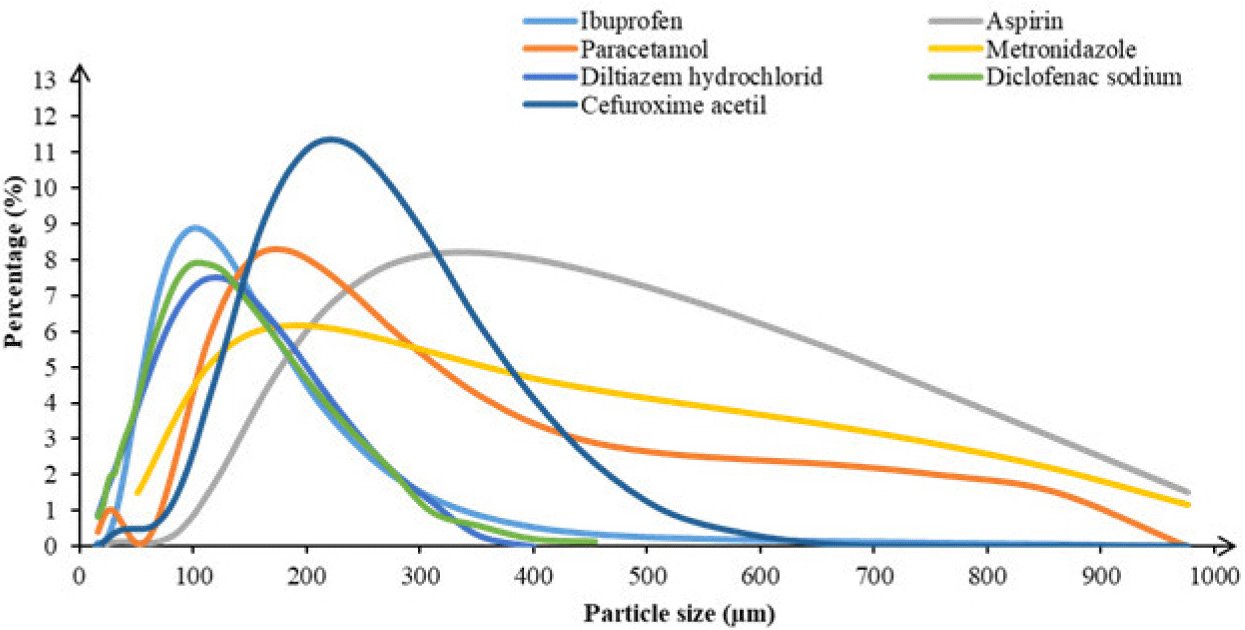
Seven model APIs were used to evaluate sticking propensity: ibuprofen (India); cefuro-xime axetil (India); aspirin (China), diltiazem hydrochloride (India), and diclofenac sodium (China) complied to USP/NF while paracetamol (China), metronidazole (China) complied to BP standards (Table 1 and Fig. 1). Other excipients used in this study include Microcrystalline cellulose PH102 (MCC PH102, Ceolus, Düsseldorf, Germany), lactose monohydrate (Sachelac® 80, Meggle, Wasserburg, Germany) and co-processed lactose monohydrate and cellulose (Cellactose® 80, Wasserburg, Germany). Additional excipients were dicalcium phosphate anhydrous (A-Tab, Berlin, Germany), pregelatinized maize starch (Starch® 1500, Colorcon, Harleysville, PA, USA), povidone K30 (PVP K30, UniClean America, Houston, TX, USA), magnesium stearate and acid stearic (France), talc (China), aerosil (Germany).
In this study, the level of sticking was defined as the amount of powder adhered to the punch surface during compression. The adhered powder were extracted by immersing the punch cup in specific solvent. The API content in the extract solution was quantified using UV spectroscopy. The specific solvents and wavelengths for each API were shown in Table 2.
Trials for preparing and compressing tablets containing one of the model APIs were conducted using the direct compression method. The compositions of each trial include the model API (40%), MCC PH102 (57%), PVP K30 (2%), and magnesium stearate (1%). For final blend preparation, each ingredient was sifted through a 35-mesh sieve prior to the mixing stage for 15 min at the speed of 100 rpm (Eweka AR403). The final blends were compressed using a standard concave punch cup (diameter 11 mm) on a rotary tablet press with a theoretical tablet weight of 250 mg. After every 50 compactions, up to a total of 300 compactions, the amount of powder adhered to the punch surface was extracted by immersing the punch cup in 50 mL of an appropriate solvent and sonicating it for 5 minutes before determining the API content using UV spectroscopy.
The melting point of APIs was determined by differential scanning calorimetery (DSC) with a Stuart SMP-10 Bibby (UK). The particle size of the model compounds was determined using a Mastersizer 3,000 particle size analyzer (Malvern, UK).
The effect of filler types and lubricants in punch sticking was investigated using the formulations presented in Table 3. Trials F1–F3 were used to investigate the effect of varying active ingredient loading percentages (e.g., 30%, 40%, 50%) on punch adhesion; Trials F4–F7 explored the impact of different filler types (e.g., MCC PH102, Sachelac® 80, A-Tab, Starch® 1500, and Cellactose® 80) on punch sticking propensity; Trials F8–F11 were designated to investigate the effect of different lubricants (e.g., magnesium stearate, stearic acid, talc, and aerosil) on punch sticking propensity.
The tablets, employing aspirin as model API, were compressed using a standard concave punch cup (diameter 11 mm) on a rotary tablet press. The weight and hardness of tablets were carefully controlled to ensure uniformity. The sticking propensity was evaluated by measuring the amount of aspirin powder adhered to the punch cup, as described above.
Coated punch (e.g., stainless steel or chromium nitride-coated steel) represents an option to reduce adhesion forces between the tablet compositions and punch surface. Chromium nitride-coated tools were chosen for their high hardness and smoothness, which reduce adhesion by minimizing surface roughness and molecular interactions. The F5 was used to investigate the effect of punch material on sticking propensity.
The shape of the punch (flat, standard concave, deep concave) was selected based on their common usage in tablet manufacturing and their varying degrees of surface contact, which were hypothesized to influence sticking.
All punches were made of standard steel material with a diameter of 11 mm. The sticking propensity was evaluated by measuring the amount of aspirin powder adhered to the punch surface.
3. RESULTS
Table 4 presents a comprehensive occurrence in the punch-sticking behavior of seven APIs. The API group comprising cefuroxime, diltiazem, diclofenac sodium, and metronidazole exhibited a low risk of sticking, in which the amount of API attached to the punch after 300 compactions not exceeding 50 µg. After 50 compactions, approximately 104.5 µg of paracetamol powder stuck to the punch, indicating considerable punch sticking. Nevertheless, there was no increasing trend in the quantity of paracetamol powder stuck to the punch during the compression process, only around 100 µg. Increasing compactions, aspirin and ibuprofen showed significant punch sticking and a progressive rise in adhesion. The number of aspirin or ibuprofen powder adhered to the punch was 133 µg or 60 µg after the first 50 compactions, respectively. These amounts increased to 345 µg for aspirin and 593 µg for ibuprofen after 300 compactions.
However, particle size could also affect punch sticking. The investigation results of the sticking tendency on two means of aspirin (aspirin with large particle size and aspirin with small particle size, namely aspirin-L and aspirin-S, respectively) showed that after 50 compactions, roughly 60 µg of Aspirin-L powder stuck to the punch. At 300 compactions, the aspirin-L stuck to the punch cup significantly increased to approximatey 595 µg. Meanwhile, there were no significant differences between the aspirin-S adhered to the punch cup when increasing compactions from 50 to 300 compactions, with levels remaining at approximately 30 µg (Table 5).
| Aspirin | Compactions | |||||
|---|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | 250 | 300 | |
| Aspirin-L | 59.57 | 86.37 | 172.48 | 236.47 | 387.36 | 593.39 |
| Aspirin-S | 20.81 | 18.21 | 21.85 | 29.14 | 19.25 | 26.53 |
In Table 6, there was a clear positive correlation between the API loading and the amount of API that adheres to the punch cup. Specifically, when the drug loading is increased from 30% to 50%, the amount of aspirin powder sticking to the punch cup increased from approximately 172 to 414 µg after 300 compactions.
| Drug loading (%) | Compactions | |||||
|---|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | 250 | 300 | |
| 30 | 113±15 | 119±6 | 134±9 | 155±22 | 163±14 | 202±14 |
| 40 | 120±15 | 152±25 | 157±19 | 170±25 | 206±18 | 244±11 |
| 50 | 172±18 | 229±52 | 253±38 | 302±21 | 363±26 | 414±14 |
The fillers exhibited varying levels of punch stikcing, as indicated by the amount of aspirin powder adhered to the punch surface, which was ranked in descending order: A-Tab (Dicalci phosphate), Cellactose® 80 (Lactose monohydrate), Sachelac® 80, MCC PH102 (Microcrystalline Cellulose PH102), Starch®1500 (Table 7). The formulation used dicalcium phosphate (A-tab) as filler, which exerted significant sticking after 300 compactions with the amount of API powder stuck to the punch was 1,062±38 µg. In contrast, the formulation used MCC or starch 1500 no significant increase in the amount of powder adhered to the punch cup as the number of compactions increased.
As shown in Table 8, the tablet containing either magnesium stearate or stearic acid had negligible adherence. The amount of aspirin powder adhered to the punch was approximately 50 µg after 300 compactions. The tablet containing talc or aerosil significantly increased the amount of aspirin powder adhered to the punch, approximately 270 µg and 300 µg, respectively.
Table 9 demonstrates that after 300 compactions, there was an increase in the amount of aspirin powder adhering to the punch when using two types of punch materials: stainless steel and chromium nitride-coated steel. Prior research had indicated that chromium nitride-coated punches, due to their exceptionally smooth texture, could minimized punch-tip adhesion by reducing chemical interaction with the APIs. However, it was observed that the chromium nitride-coated steel punch did not alleviate punch sticking as much as anticipated.
| Punch material | Compactions | |||||
|---|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | 250 | 300 | |
| Stainless steel | 172±18 | 229±52 | 253±38 | 302±21 | 363±16 | 414±14 |
| Chromium nitride-coated steel | 181±17 | 238±15 | 256±28 | 312±14 | 357±13 | 375±12 |
As shown in Table 10, there was a significant difference in the amount of aspirin powder adhering to the punch depending on the punch geometries (flat face, standard concave cup, and deep concave cup). The more concave the punch cup, the less aspirin powder stuck to the punch cup, approximately 340±20 µg and 414±14 µg, respectively. Sticking tendency occurs most on punches with flat face, approximately 449±30 µg aspirin powder adhered.
4. DISCUSSION
The result of investigation on 7 model drugs with different physicochemical properties showed that the group of APIs with a higher melting point and smaller particle size are less likely to adhere to the punch cup. The variations in the interaction force between API and excipient may be one of the factors dictating the rate and severity of adhesion in these cases. API-API cohesion was found to be more significant than the cohesion between API and the other ingredients. The API with a high melting point (i.e., Metronidazole, diltiazem, diclofenac, or cefuroxime) exhibited a low risk of sticking, classified as type I sticking behavior, as described of [8], due to the weak attraction between the API molecules and the punch material.
In contrast, aspirin or ibuprofen can cause type II sticking, characterized by progressive adhesion development. API-API cohesion is greater than that of between API-inactive ingredients. During compression, the API layer on the punch will grow thicker [8]. Ibuprofen has a low melting point (76.5°C) (Fig. 2) and is known to create eutectics with stearic acid, which lowers the substance’s melting point and increases its sticking tendency [9]. These findings best fit to those reported by [9].
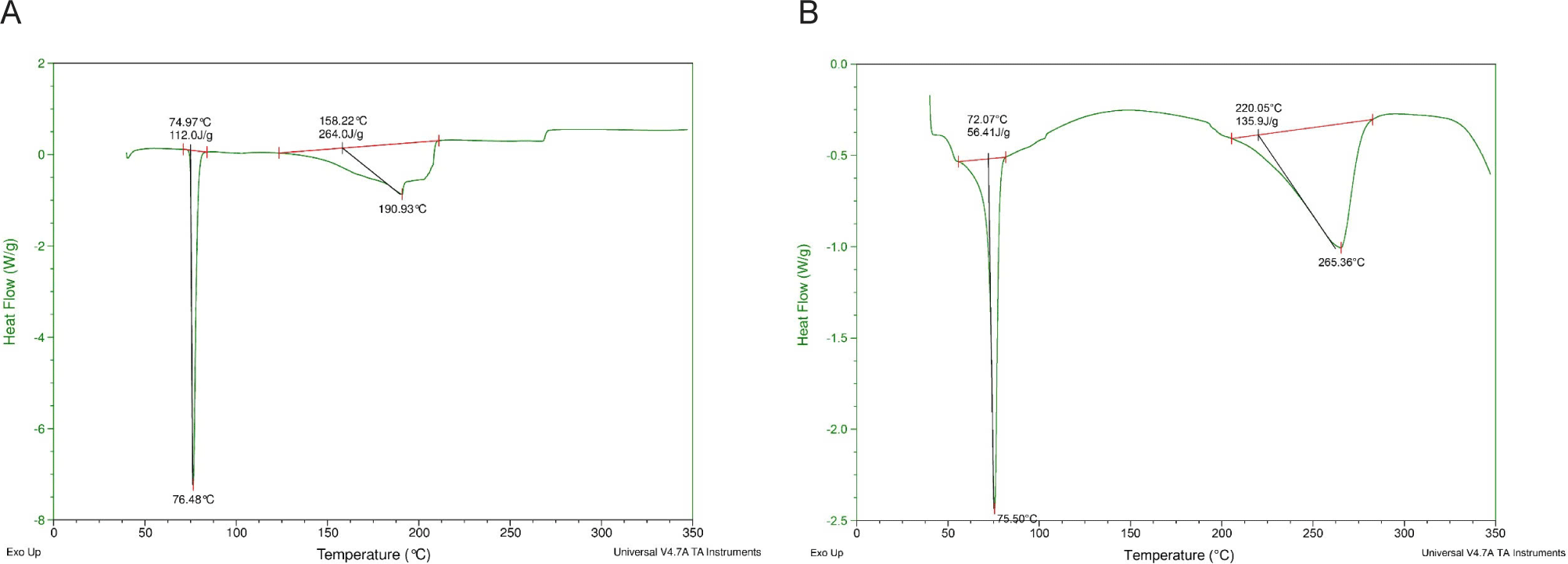
However, the melting point is not the sole element causing the punch sticking. The result of this study proved that aspirin had different sticking behavior compared to those of Cefuroxime, even though these two APIs have quite similar melting points (131°C and 135°C, respectively). Particle size also appears to affect punch sticking, with smaller particle size having lower adhesion tendencies. For example, the APIs with small size around 100 µm of diltiazem hydrochloride or diclofenac sodium (with mean sizes 104 µm and 96 µm, respectively), showed low adhesion tendencies. Our study revealed that aspirin with small particle sizes (178 µm) (namely Aspirin-S) had significantly lower adhesion tendencies compared to that of aspirin with large particle sizes (361 µm) (namely Aspirin-L). These findings differed from those reported by [8], who observed that larger particle sizes exhibited lower adhesion tendencies.
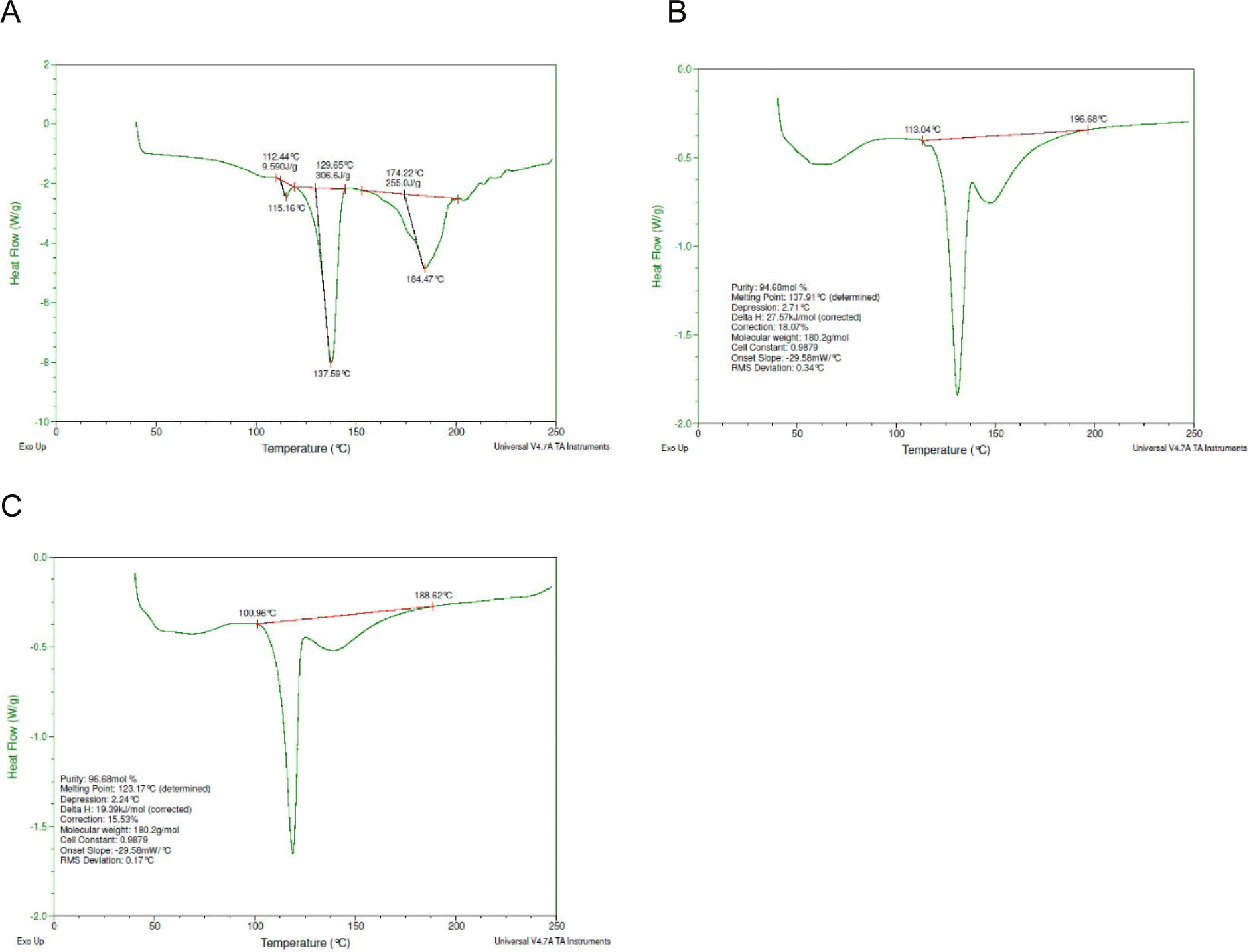
The DSC thermograms and X-ray diffraction analysis (XRD) graphs of the raw materials and the mixture of APIs with other ingredients were used to analyze the punch-sticking mechanism for both aspirin types. The powder adhered to the punch was also analyzed using the same techniques. The DSC thermogram provided evidence of a shift in melting point, which suggested changes in the crystalline structure of the API. The DSC thermogram of aspirin showed decreased melting points after compression, especially in powder adhering to the punch surface. The DSC thermograms of Aspirin-S and Aspirin-L revealed that their melting points were the identical (138°C), while the melting point of the adhered powder had decreased (123°C) (Fig. 3). The change in the crystal network structure of aspirin caused the shift in melting point. These changes could lead to increasing in molecular mobility at low temperatures, enhancing the adhesion to the punch surface.
XRD graphs of aspirin before and after compression showed a significant loss of the crystalline typical peaks associated with its raw form. XRD graphs of Aspirin-L showed that the crystal network structure of Aspirin-L changed after the compression, the typical XRD peaks of aspirin did not appear in the graphs [10] (Fig. 4), indicating that the compression exerted the specific structural changes, which may have been caused by compression force [11,12].
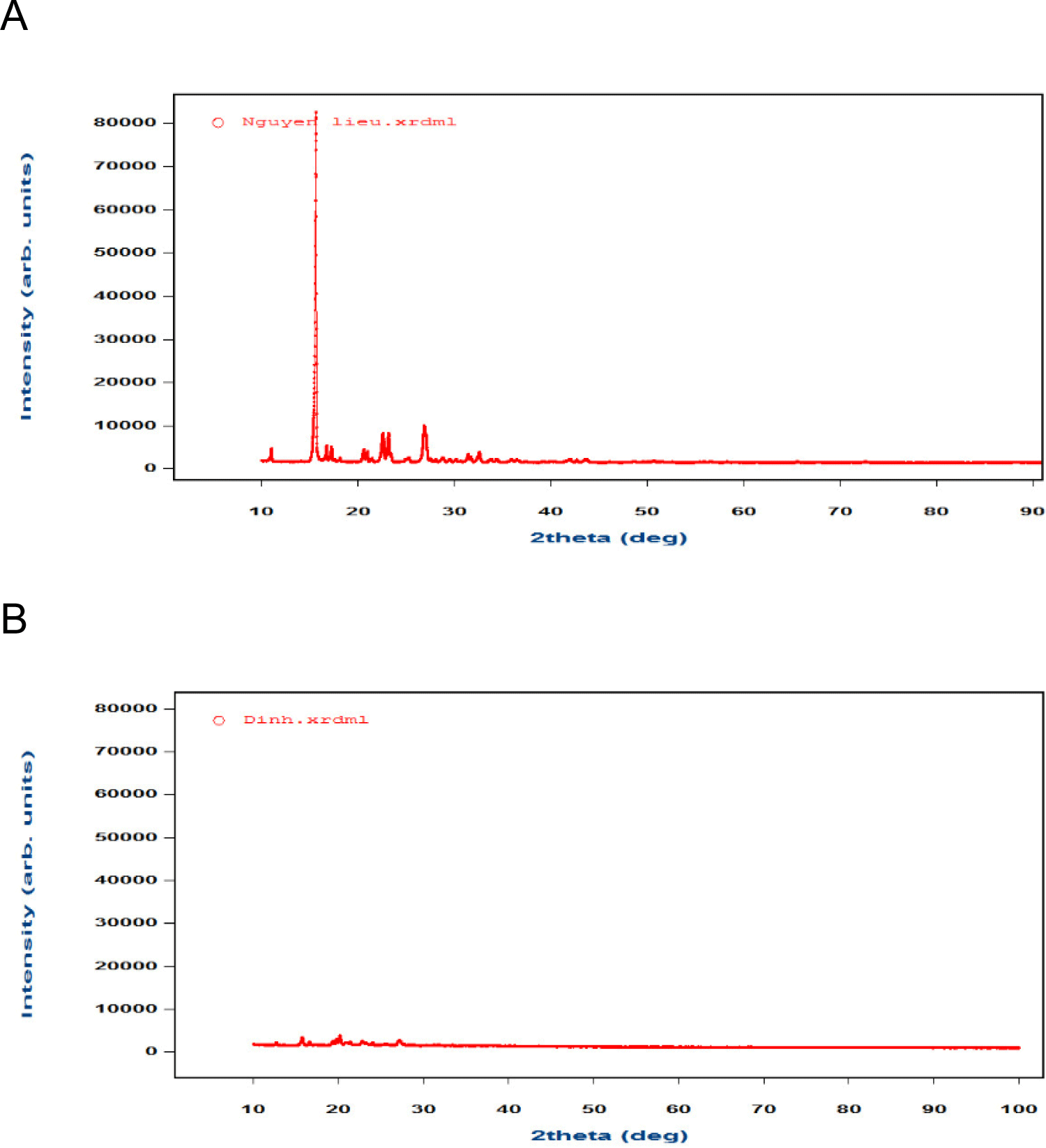
The tablet’s hardness increased from 20 newtons to 80 newtons, resulting in a decrease in the intensity of typical peak of Aspirin. However, the typical peak of aspirin-L could be seen on the XRD graphs (Fig. 5) proved that aspirin-L with adhesion to the punch cup did not retain its original crystal network structure. This reduction in crystallinity implied structural changes under compaction pressure, likely due to the transformation from a crystalline to the partially amorphous form. Such transformations of aspirin could increase surface energy and adhesion potential, which are closely related to tablet adhesion.
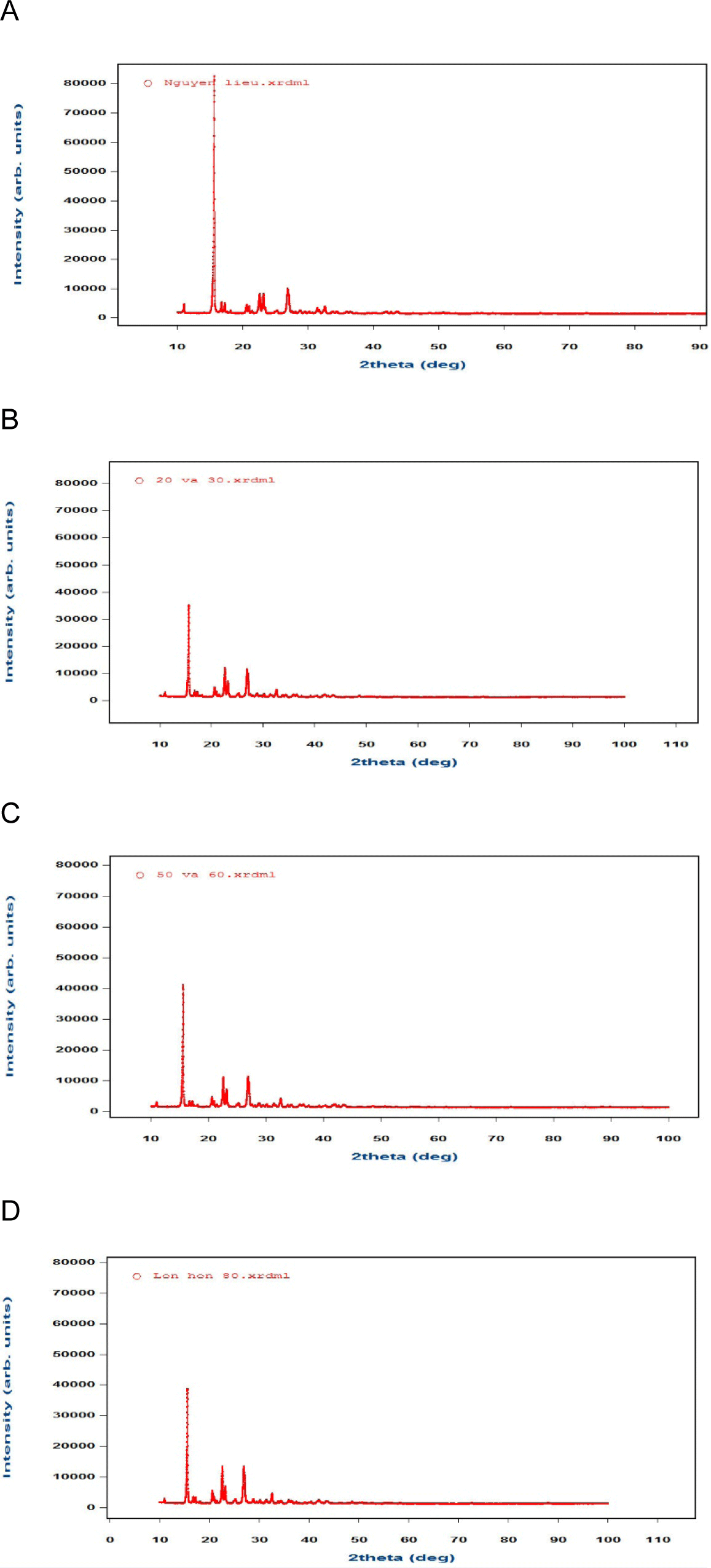
Consequently, low melting points and/or large particle mean sizes were favorable circumstances for raising the cohesive force of the drug substance on the metal surface.
Another factor that could cause punch sticking is the API loading. The more API loading, the more sticking tendency of aspirin developed. This could be attributed to the high loading of API particles in the die before compaction, leading to a more significant amount of particles encountering and adhering to the punch face during compaction. As a result, formulations with a lower loading of API exhibited a reduced tendency for adhesion. This finding is consistent with a previous study by [13].
The punch sticking was the interplay of intermolecular forces between the formulation components and the punch surface [14]. This phenomenon occurred when the interfacial force between the punch and API significantly exceeded the cohesive forces between API molecules and the API-excipient interactions. The development of the extent and rate of adhesion depends on the relative strength of API-API and forces of API-excipient. In the context of the fillers investigated in this study, adhesion was categorized as Type II adhesion, as propose of [8], and the amount of API powder stuck to the punch at the 300 compactions was two-fold compared with that of 50 compactions. Dicalcium phosphate, an inorganic filler, primarily interacted with APIs via weak Van der Waals forces. The organic excipients (Starch®1500, MCC PH102, Cellactose® 80, Sachelac® 80) had the electrostatic interactions predominate.
Consequently, the API-dicalcium phosphate (A-tab) interaction was significantly weaker than that of other excipients, resulting in the most severe sticking phenomenon among the five investigated filler excipients [15,16]. Sachelac® 80 comprises α-lactose monohydrate crystals that undergo fracture deformation during compression under high pressure, resulting in a porous structure, reducing the API-excipient [17] internacaction, MCC PH102 can form hydrogen bonds between adjacent hydroxyl groups within its cellulose molecule, imparting strong and cohesive forces between particles in the tablet [18]. Additionally, MCC PH102 possesses high compressibility and plastic deformation under compression forces. Therefore, the extent of fracture of α-lactose monohydrate is greater than that of MCC PH102. Starch®1500 is a modified starch and exhibits elastic deformation [19]. In this case, the API-excipient interaction played a bigger role than that of MCC PH102, leading to a low degree of stickiness [6].
The effect of filler excipients in tablet formulations significantly impacted on the punch sticking propensity. It is crucial to consider the physical interactions and the formation of physicochemical bonds between the mixture of fillers and APIs to predict the potential for punch sticking when selecting filler excipients.
Lubricants help to reduce friction between compositions and tooling during compression, leading to reduced punch sticking. Magnesium stearate works as a lubricant by producing a low-shear strength film between the die wall and powder, hence reducing friction. It also has anti-adhesive and flow-enhancing properties, which prevent tablets from sticking to the die wall and to punch surfaces, providing tablet uniformity [20]. Aerosil is a glidant excipient that increases the fluidity of powder mass by reducing friction between powders through filling voids on the surface of solid particles [21]. This leads to a decrease in the number of contact points between particles, reducing cohesive force during compression. However, aerosil did not reduce friction between particles and the punch surface, which could lead to significant punch adhesion [5]. Talc, another slippery excipient, reduced friction of particle-particle and particle-punch and die, resulting in less stickiness [22,23]. However, the lubricating ability of talc is lower than that of magnesium stearate, which could result in high levels of stickiness when used in formula.
The chromium nitride-coated punches had a very smooth texture, reducing punch-tip adherence due to the low chemical interaction with the APIs [24,25]. However, it was found that the chromium nitride-coated steel punch did not eliminate punch sticking at low speeds. The electrostatic charge occurring during the mixing or die-filling processes (or both) may cause sticking. It was hypothesized that at high compaction speeds, the affinity of particles to each other may be stronger than that of the electrostatic interaction between the surface and the powder particles, leading to reduced punch sticking. Moreover, the elevated temperature during compression can weaken the adhesion forces (such as Van der Waals forces), reducing the powder’s tendency to adhere to the chromium nitride-coated surface. High compression speeds generate significant compaction pressure that would help the particles consolidate rapidly, leaving fewer particles at the surface interacting and adhering to the punch. Therefore, high compaction speeds should be conducted better eliminate the effect of punch material on sticking propensity.
Flat punches exhibited the highest degree of powder sticking, whereas deep concave punches demonstrated the lowest adhesion. This phenomenon could be attributed to variations in the density distribution within the tablet caused by the punch geometry. In flat-faced tablets, the compaction force led to localized stress concentrations, resulting in a high-density region in the central of lower half of the tablet. Conversely, the low-density region at the interface with the punch reflected weak interparticle forces as the degree of punch curvature increases (i.e., standard concave, deep concave), the axial and lateral movements of the compressed material development, potentially contributing to the formation of those regions. Cupped punches typically exhibited high-density zones near the die wall and around the tablet periphery, with a lower-density central core [2,9]. In these low-density areas, the cohesive forces between particles weaken, facilitating adhesion to the punch surface. Consequently, tablets pressed with flat punches demonstrated a higher propensity for particle sticking than those pressed with cupped punches due to a larger low-density area at the punch interface.
5. CONCLUSION
The physicochemical properties of the API (melting point, particle size) play a significant role in the propensity for punch sticking. APIs with high melting points tend to exhibit low punch-sticking tendencies. Small API particles and low API loading formulations reduce the risk of sticking. Inorganic filler excipients, such as dicalcium phosphate, generally tend to cause punch sticking, in contrast to organic fillers like MCC and starch. Dicalcium phosphate (A-tab) should be avoided in high-sticking risk formulations. Meanwhile, MCC PH102 and Starch 1500 can reduce sticking tendency.
Magnesium stearate, a commonly used lubricant, has effectively reduced punch sticking. It should be prioritized in formulations prone to sticking due to its ability to form a low-shear-strength film on the punch surface that reduces adhesion. The type of punch coating can significantly impact on sticking behavior. This study showed that chromium nitride-coated steel may not exhibit a distinct advantage over stainless steel in terms of punch-sticking mitigation, particularly for formulations containing high-melting-point APIs. Therefore, chromium nitride-coated tools should be used at high compaction speed to reduce punch sticking. Flat punches should be avoided in formulations with high sticking tendencies. In contrast, standard and deep concave punches provide a larger contact area and a more uniformed distribution of compaction forces, potentially reducing the risk of adhesion between the formulation and the punch surface.
