1. INTRODUCTION
Third-degree atrioventricular block, which occurs when no atrial electrical impulses reach the ventricular conduction system, is a cardiovascular emergency with high morbidity and mortality [1]. This condition often arises from various causes, including acute coronary syndrome, cardiomyopathy, electrolyte imbalances, infiltrative cardiac diseases, medication side effects, and metabolic disorders, especially in patients with end-stage renal disease (ESRD) [1,2]. In ESRD, electrolyte imbalances, notably hyperkalemia and abnormal calcium and phosphorus metabolism, can increase the risk of developing atrioventricular block, requiring close monitoring and management to prevent severe cardiac complications [3]. The clinical presentation of third-degree atrioventricular block often includes symptoms of bradycardia, syncope, and in severe cases, cardiac arrest, making timely intervention critical [4].
This case report emphasizes the important relationship between mineral bone disease and cardiovascular complications in hemodialysis patients, particularly the emergence of third-degree atrioventricular block, likely caused by metastatic calcification [2]. The patient, a 43-year-old woman with ESRD, represents the significant cardiovascular risks arising from metabolic disorders associated with renal failure, despite standard treatments for calcium and phosphorus abnormalities. Her recovery from third-degree atrioventricular block to sinus rhythm following a 10-day period of temporary transvenous pacing highlights the importance of timely therapies to manage such serious cardiac complications, which can result in better patient outcomes even in severe cases.
Globally, several studies have reported cardiac calcification causes by mineral bone disease in dialysis patients [5–8]. However, the successful treatment of atrioventricular block in ESRD patients has been sparsely documented, with scattered case reports from around the world [9,10]. We present a case of third-degree atrioventricular block reversal with temporary transvenous pacing that we had not encountered in clinical practice or found in any publications.
2. CASE REPORT
A 43-year-old woman living in Thai Nguyen province presented to Thai Nguyen National Hospital with recurrent episodes of fatigue and dizziness. She was a farmer who had a 6-year history of ESRD that required regular hemodialysis three times per week due to hypertension. Her medical history was complicated, including hypertension, heart failure, chronic co-infection with hepatitis B and C, and secondary hyperparathyroidism. She reported a recent fall that resulted in a 2-centimeter laceration on her forehead due to fatigue and dizziness six days prior to this hospital admission. Notably, she stated that she had not used digitalis or beta-blockers.
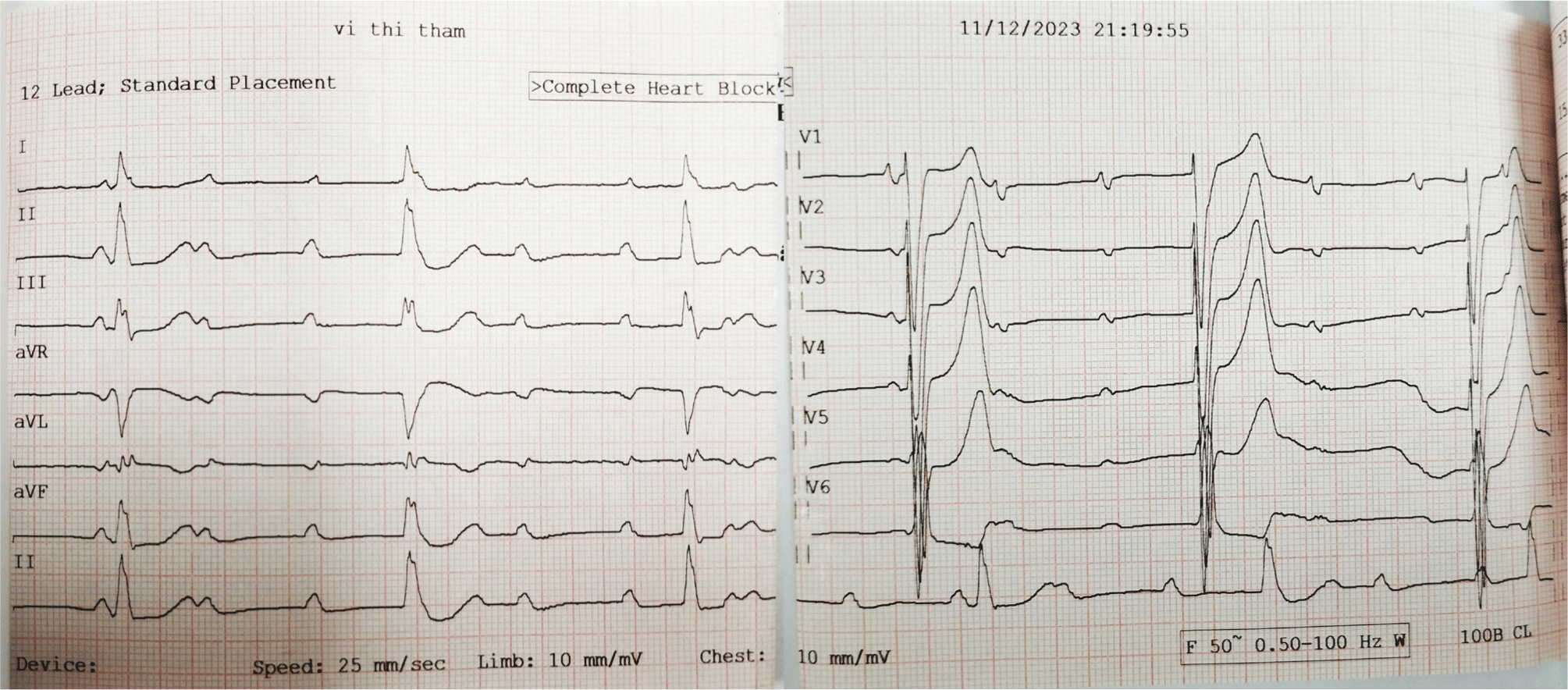
Upon examination, the patient presented with a blood pressure of 100/60 mmHg and exhibited dyspnea, with a respiratory rate of 25 breaths per minute. Her heartbeat was irregular, approximately 30 beats per minute. Pulmonary examination revealed decreased vesicular breath sounds bilaterally. Initial laboratory results indicated a serum creatinine level of 781.40 µmol/L (eGFR: 5 mL/min/1.73 m²), alongside elevated serum parathyroid hormone (PTH) levels higher than 265.00 pmol/L, a serum phosphorus level of 3.17 mmol/L, a serum total calcium level of 2.92 mmol/L, and a serum potassium level of 5.35 mmol/L. An electrocardiogram (ECG) showed a third-degree atrioventricular block with a ventricular rate of 33 beats per minute, as well as signs of left ventricular hypertrophy and myocardial ischemia (Fig. 1). A transthoracic echocardiogram revealed severe mitral stenosis with significant mitral valve regurgitation (Wilkins score of 9), mild aortic valve stenosis, severe aortic regurgitation, and severe tricuspid regurgitation, while the left ventricular size and systolic function remained normal (ejection fraction of 73%). An abdominal ultrasound displayed bilateral renal cysts and chronic kidney disease. A frontal chest X-ray appeared normal, and a head CT scan was not performed at the time of evaluation.
The patient’s differential diagnosis included a third-degree atrioventricular block associated with metastatic calcification in the cardiac conduction system, which is common in patients with ESRD on hemodialysis and secondary hyperparathyroidism. Severe mitral stenosis and substantial mitral valve regurgitation were also considered as contributory factors. Another potential diagnosis was subarachnoid bleeding from a traumatic brain injury, which could result in bradycardia complications. The final diagnosis confirmed the presence of a third-degree atrioventricular block due to metastatic calcification in the context of the patient’s ESRD and hemodialysis treatment. The prognosis for this patient is cautiously optimistic. Timely interventions, starting with temporary transvenous pacemaker placement, in conjunction with ongoing management of her renal and cardiac conditions, may help to stabilize her heart rhythm and improve her overall health. Nonetheless, regular evaluation of her ESRD and associated complications is crucial to her long-term prognosis.
To address the critical atrioventricular block, an urgent implantation of a temporary transvenous pacemaker was performed, programmed to maintain a heart rate of 80 beats per minute for 10 days. Pharmacologic therapy included cefoperazone (1,000 mg twice a day for 12 days), erythropoietin (2,000 IU three times weekly), and antihypertensive medicine (cilnidipine 5 mg daily). Additionally, the patient was given vitamin B1, B6, and B12 supplements, as well as diazepam (5 mg once a day for 12 days). The patient continued to receive hemodialysis three times per week through a right arm arteriovenous fistula. A dietary consultation was offered to assist her in following a low-phosphorus, low-sodium diet.
Considering the potential risks associated with both temporary and permanent pacemaker implantation procedures, and the patient’s susceptibility to requiring a permanent cardiac pacemaker implantation, a decision was made to refrain from implanting a permanent pacemaker at that time. Instead, continuous ECG monitoring was maintained. On the tenth day post-temporary transvenous pacemaker placement, the patient’s rhythm reverted to sinus rhythm. Consequently, the temporary transvenous pacemaker was removed, and follow-up ECGs were conducted on the first and second days thereafter, as well as one and a half months later (Figs. 2 and 3).
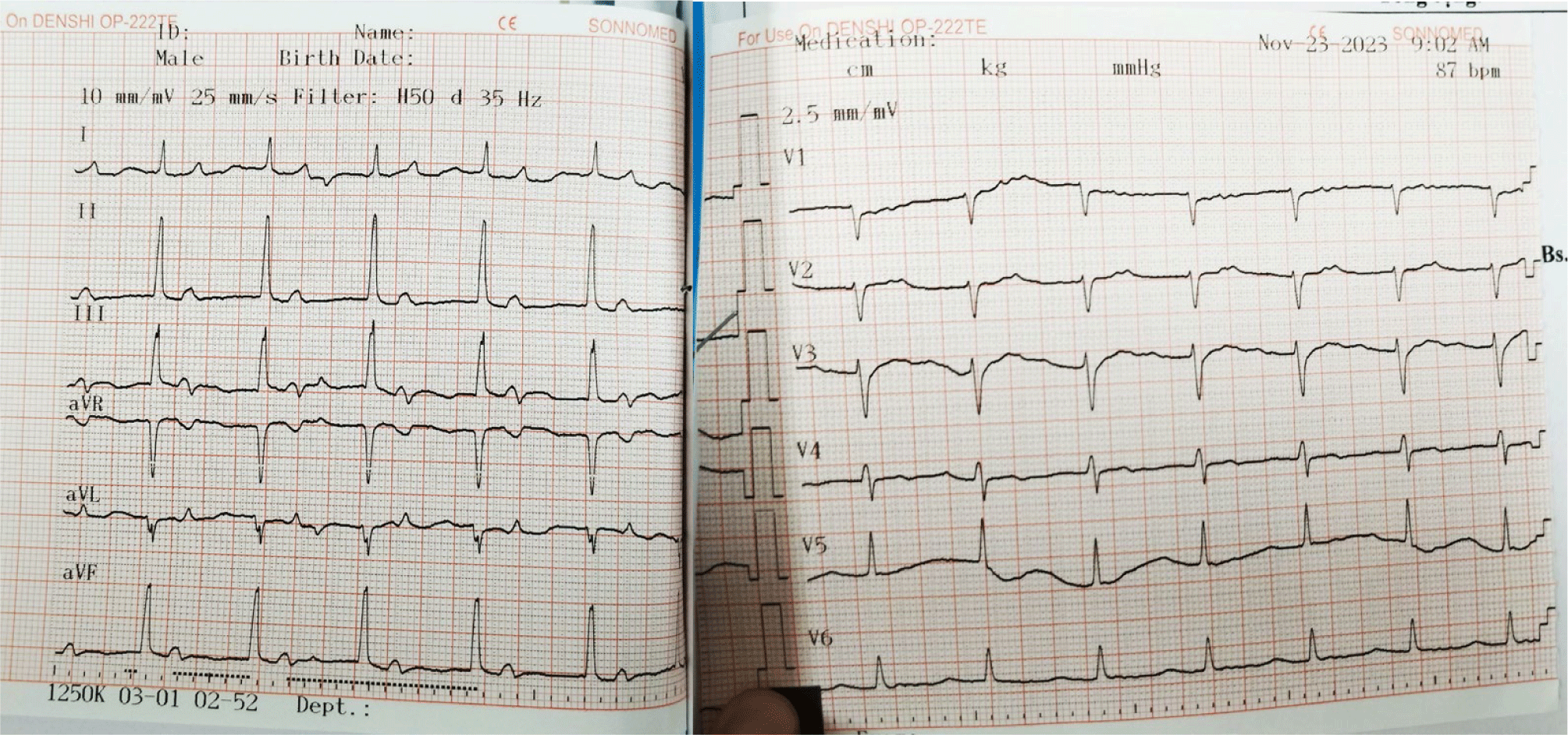
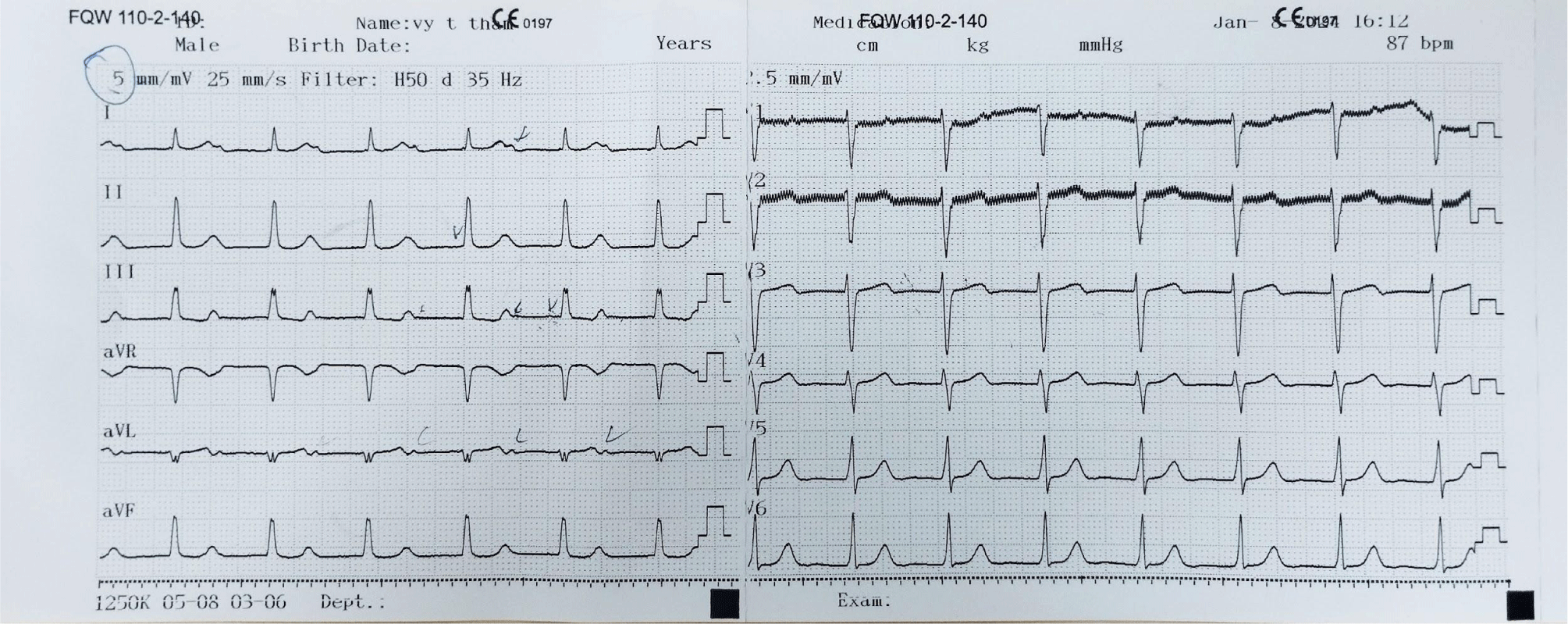
The ECG following the removal of the temporary transvenous pacemaker revealed sinus rhythm at a rate of 97 beats per minute, first-degree atrioventricular block with a PR interval of 0.23 seconds, and signs of left ventricular hypertrophy.
A month and a half later, the patient still maintained sinus rhythm and exhibited first-degree atrioventricular block. Her serum potassium level ranged between 3.5 and 5.0 mmol/liter, and her serum creatinine level was at 636.31 µmol/L (eGFR: 7 mL/min/1.73 m2). A head CT scan performed at this time revealed no evidence of brain or meningeal hemorrhage. However, the presence of 5 mm-sized low-density foci in the pontine parenchyma without surrounding edema suggests a cerebral defect.
For a detailed evaluation of reporting standards following CARE Case Report Guidelines [11].
3. DISCUSSION
In patients with ESRD undergoing hemodialysis, mineral bone disease is a significant concern despite efforts to address abnormalities in plasma calcium and phosphorus concentrations [2]. Metastatic calcification, the accumulation of calcium in normal soft tissues, is a common consequence of these metabolic disorders. Cardiac conduction abnormalities are among the clinical manifestations of this condition.
Several authors have reported cardiac calcification in dialysis patients. Forman et al. revealed that mitral annular calcification occurs more commonly in patients on dialysis for a long period [5]. In addition, Varma et al. reported that mitral annular calcification occurred in 10%–50% of ESRD patients [6]. This is significant because mitral annular calcification is linked to a high frequency of conduction abnormalities. Nair et al. discovered mitral annular calcification was present in 87% of patients with symptomatic bradyarrhythmia [7]. While the term “mitral annular calcification” implies that the calcification is limited to the mitral annulus, research using autopsied and surgical samples has shown that the calcification extends into the left ventricle, mitral leaflets, papillary muscle, chordae tendineae, and left ventricular outflow tract. Occasionally, the aorto-mitral curtain continuously calcifies, extending up into the aortic valve [8].
While it is conceivable that the patient experienced a third-degree atrioventricular block due to metastatic calcification in the cardiac conduction system, the current evidence is insufficient to confirm this diagnosis. The patient’s symptoms could also be attributed to severe mitral stenosis and severe mitral valve regurgitation, conditions that may not necessarily be linked to calcification. Additionally, given the history of subarachnoid bleeding following traumatic brain injury, bradycardia could be a potential manifestation. Unfortunately, a head CT scan was not conducted at the time of evaluation. Subsequent to a head CT scan performed at the one-and-a-half-month mark, no indications of brain or meningeal bleeding were observed. The presence of foci of decreased density in the pontine parenchyma, approximately 5mm in size, without surrounding edema, suggests a cerebral defect.
We hypothesized that elevated PTH levels might be the underlying cause of calcium accumulation in the cardiac conduction system. Previous studies indicated the reversal of atrioventricular block after parathyroidectomy [9,12]. Another case report involving complete atrioventricular block attributed to tertiary hyperparathyroidism also discussed parathyroidectomy as a potential intervention to prevent this condition [10]. While our findings demonstrate that a temporary transvenous pacemaker can convert third-degree atrioventricular block to first-degree atrioventricular block within ten days, there is insufficient evidence to support the prolonged use of a temporary transvenous pacemaker in long-term hemodialysis patients for this duration [4,13]. Additionally, concerns exist regarding the potential progression of atrioventricular block after the removal of the temporary transvenous pacemaker. Typically, a transition to a permanent pacemaker would be considered after a week of persistent pacing necessity, with the procedure commonly scheduled for the seventh day following temporary pacemaker implantation [14]. The decision to defer permanent pacemaker implantation on the seventh day was driven by our desire to avoid subjecting the patient to consecutive procedures, as the associated complications could exacerbate her condition. By the tenth day, the patient had reverted to sinus rhythm on ECG, eliminating the need for a permanent pacemaker.
In our patient, we presumed that her atrioventricular block could be reversed due to better control of serum PTH, phosphate, and calcium levels. Still, treatment recommendations are supported by inadequate evidence, and therapeutic failures are common. The extensive list of treatments, which include phosphate binders, calcium-sensing receptors, bisphosphonates, calcimimetics, magnesium, vitamin K, emergency parathyroidectomy, plasma exchange, and dialysis treatment optimization, indicates unclear efficacy [15–17]. To prevent the progression of the patient’s atrioventricular block, in addition to implanting a temporary transvenous pacer and permanent pacemaker, parathyroid surgery may be considered the most effective option. This is due to its potential to reduce vascular calcification-promoting factors by decreasing phosphorus levels, calcium-phosphorus product concentration, and PTH levels. Despite the possibility of late regression of vascular lesions occurring more than 12 months post-parathyroidectomy, parathyroid surgery remains a promising approach for controlling the advancement of vascular calcification [18]. However, the current study did not confirm the potential correlation between the degree of normalization in blood calcium, phosphorus, calcium-phosphorus product, and PTH abnormalities and the extent of improvement in coronary calcification.
This case report also reveals both strengths and limits in understanding the association between mineral bone disease, cardiac conduction issues, and treatment approaches in ESRD patients undergoing hemodialysis. A key strength is its primary focus on the clinical significance of metastatic calcification in the cardiac conduction system, which implies the link between mitral annular calcification and cardiac arrhythmias, and demonstrates the effective use of a temporary transvenous pacemaker to manage the patient’s third-degree atrioventricular block. However, the study has a few limitations, including insufficient evidence to conclude that metastatic calcification was the sole cause of the atrioventricular block, as other alternative factors were not thoroughly investigated. In addition, there is a lack of long-term data on the effects of prolonged temporary pacing, and the decision to postpone permanent pacemaker placement raises concerns about the potential for recurrent arrhythmia. Overall, while the paper provides useful information, it also highlights the complexity of detecting and treating cardiac complications in this vulnerable population, indicating the need for further research to refine approaches.
The patient found the treatment both reassuring and challenging. The diagnosis of third-degree atrioventricular block and the need for a temporary pacemaker raised worries about her health and potential complications. However, the support from medication and nutritional consultations made her feel hopeful about her outcome. Clear communication from the healthcare team about managing her renal disease and secondary hyperparathyroidism provided the patient a sense of control over her health. While the prospect of ongoing monitoring and additional treatments was concerning, she expressed deep appreciation for the healthcare team’s efforts to restore her cardiac rhythm and enhance her quality of life.
4. CONCLUSION
Patients undergoing long-term hemodialysis are at a higher risk of developing cardiac conduction abnormalities. When an atrioventricular block coincides with calcification of the mitral valve annulus and elevated PTH levels, it may be necessary to conduct repeated ECG tests to identify more severe conduction issues. For patients experiencing an atrioventricular block, the prompt placement of a temporary transvenous pacemaker can be a critical and potentially life-saving intervention. In light of these factors, early consideration of parathyroidectomy is recommended to help prevent the progression of atrioventricular block.