1. INTRODUCTION
Glycated hemoglobin (Hemoglobin A1c, HbA1c) is continuously formed by a covalent bond connection of glucose to the N-terminal valine of the hemoglobin chain to form ketoamine. A higher concentration of glucose caused forming more glycated hemoglobin molecules. Therefore, the concentration of HbA1c in the erythrocytes reflects the mean plasma glucose concentration erythrocytes in about 120 days, which is equivalent entire to the life span of the red blood cell [1]. The HbA1c test was guided by the American Diabetes Association to diagnose and monitor diabetes treatment. It is also widely used in clinical practice and is one of the most vital criteria for clinicians to evaluate the effectiveness of diabetes treatment. Thus, quality control of HbA1C assay consists of internal and external quality assessment (EQA) as a requirement according to ISO 15189: 2012 [2]. In these requirements, the level of quality control materials shall be at various concentrations including low, normal, and high. Moreover, the high level of HbA1C is important because it aids clinicians to make treatment decisions. In the Standards of Medical Care in Diabetes of the American Diabetes Association, the criteria for screening and diagnosis of diabetes classify in three ranges: normal (<5.7%), prediabetes (5.7% - 6.4%), and diabetes (≥ 6.5%) [1]. Therefore, one of the most important characteristics of EQA samples is the matrix as the same as patient samples, so collecting donor blood from diabetes patients may violate ethics because anemia is one of the diabetes complications. According to Vietnamese regulation, the blood donors must be people with non-chronic diseases, so the packed red blood cells mainly originated from non-diabetes donors caused the lack of raw external quality control materials [3]. The key solution is actively increasing HbA1c concentration at prediabetes (5.7%-6.4%) and diabetes (≥ 6.5%) from non-diabetes donor blood to ensure quality control in EQA participants [1]. Hence, this research proposes the optimal procedure to increase HbA1c concentration at definite medical decision levels.
2. MATERIALS AND METHOD
Packed red cells that were screened non-reactive with HBV, HCV, HIV, malaria, and Syphilis, were withdrawn from donors in ChoRay Blood Transfusion Center and transported to Quality Control Center for Medical Laboratory within 72 hours in the cooler box with ice packages. In this study, the packed red cells were collected and transported immediately to QCC UMP 6 hours after blood bags were produced. The characteristics of three blood bags sequentially are blood group “O”, “A’, “B”; Rhesus Positive; donor date: 6 Jan 2021; manufacture date: 08 Jan 2021.
All reagents include Ringer solution, BAGPM, PBS, Salin, Alsever solution, anhydrous NaCl, anhydrous KCl, CaCl.6H20, Sodium Lactate solution 60%, anhydrous glucose was supplied by Sigma (analytical grade). These reagents were weighed by Sartorius Model TE214S Talent Analytical Balance, laboratory glassware (Duran®). The RBCs suspensions were incubated at 2°C - 8°C in cool shelves of Panasonic top freeze refrigerator, at 22°C - 24°C in the laboratory with air conditioner, and 37°C in LEEC classic incubator model C157. The incubated temperatures were observed by glass thermometers that have been calibrated in an approved laboratory for calibration.
This study has been approved by the Ethics Committee at the University of Medicine and Pharmacy at Ho Chi Minh City. The study was carried out from October/2019 to January/2021 at Quality Control Center for Medical Laboratory under the Ministry of Health, University of Medicine and Pharmacy at Ho Chi Minh City. The HbA1c concentration of the packed red cells was determined and samples that met non-diabetes criteria were proceeded to the next steps [1]. After that, the packed red cells were washed with isotonic saline according to the following procedures.
Sample size: three blood bags were collected from three different healthy blood donors.
RBCs were washed three times by isotonic saline with a ratio of 1:2 and centrifuged at 5°C at 2000xg for 15 minutes each time. In each experiment, HbA1c concentration was measured from 1-mL vials containing RBC suspension in specific solutions, which were then discarded according to regulatory guidelines. After washing three times with isotonic saline, the washed red cells were diluted to one part two with Ringer, BAGPM, Saline, PBS. The initial concentration of HbA1c in four solutions was determined on Beckman Coulter AU480 shows as day 0 in Table 1 before incubating in the next experiments. All RBC: solution ratios across the manuscript are “vol/vol ratio”.
Phase 1: studying the best buffer solutions for increasing HbA1c concentration. There are four solutions included Ringer (1 mM CaCl2, 5 mM KCl, 145 mM NaCl, 2 mM MgCl2, 10 mM HEPES/NaOH, 100 mM glucose), BAGPM (101.4 mM NaHCO3, 14.3 mM Na2CO3, 1mM Na3PO4, 0.5 mM mannitol, 1 mM adenine, 100 mM glucose), Saline (150 mM NaCl, 100 mM glucose), Phosphate buffer saline (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, 100 mM glucose) [4-4]. Washed red cells were dispersed in vials contained one of four preserved solutions above with the vol/vol ratio 1:1 and incubated at 37°C for 15 days.
Phase 2: studying the optimal temperature for increasing HbA1c concentration. The chosen buffer solution in phase 1 was used to look for the suitable temperature including 2°C – 8°C, 22°C – 24°C, 37°C.
Phase 3: studying the suitable glucose concentrations on increasing HbA1c concentration. Three adjusted glucose concentrations in the chosen buffer solution at 100 mM, 305 mM, and 500 mM were observed in 15 days.
In the experiment, the concentrations of both HbA1c and total hemoglobin are determined in triplicate on Beckman Coulter AU 480 analyzer once per day in 15 days. The HbA1c/total hemoglobin ratio is expressed as the percentage of HbA1c. In this method, total hemoglobin is measured via the conversion of all hemoglobin derivatives into alkaline hematin in the alkaline solution of a non-ionic detergent, and HbA1c is measured in a latex agglutination inhibition assay. The assay was verified according to the guideline of CLSI EP15 A3 with acceptable results [8].
All statistical analyses were performed using Stata 14.0. One-way ANOVA was used to analyze differences among the samples in each condition and across solutions/conditions. The differences between the time points from day 0 were evaluated by One-Way Repeated Measures ANOVA. All our data and figures are mean value ± SD.
3. RESULTS
In phase 1, the ratio between washed RBCs suspension and one of the following solutions: Ringer, BAGPM, Saline, and PBS with 100 mM glucose was 1:1 and incubated in 15 days.
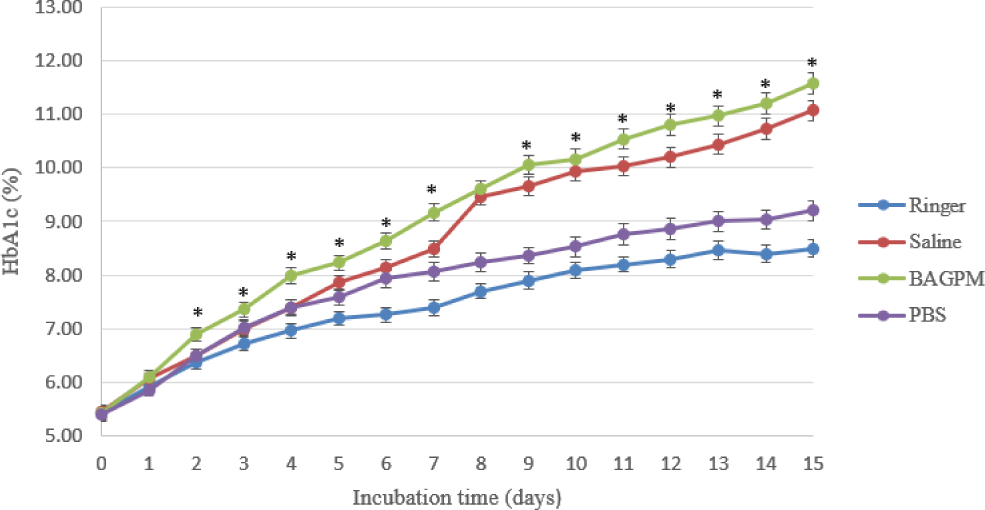
In Table 1, the rate of increasing HbA1c concentrati on in the BAGPM solution was the fastest than the other achieve 11.57±0.2% in 15 days at 37°C. On the other hand, the concentration of HbA1c in Ringer solution was at the peak of 8.5±0.16% at the same time. With Saline and PBS solution, the concentration of HbA1c increased sequentially 11.07±0.18% and 9.2±0.19% in the research time.
In phase 2, the BAGPM solution was chosen for the experiments about the effect of temperature on HbA1c concentrations over 15 days at glucose 100 mM and the effect of glucose concentration on HbA1c concentration over 15 days at 37°C.
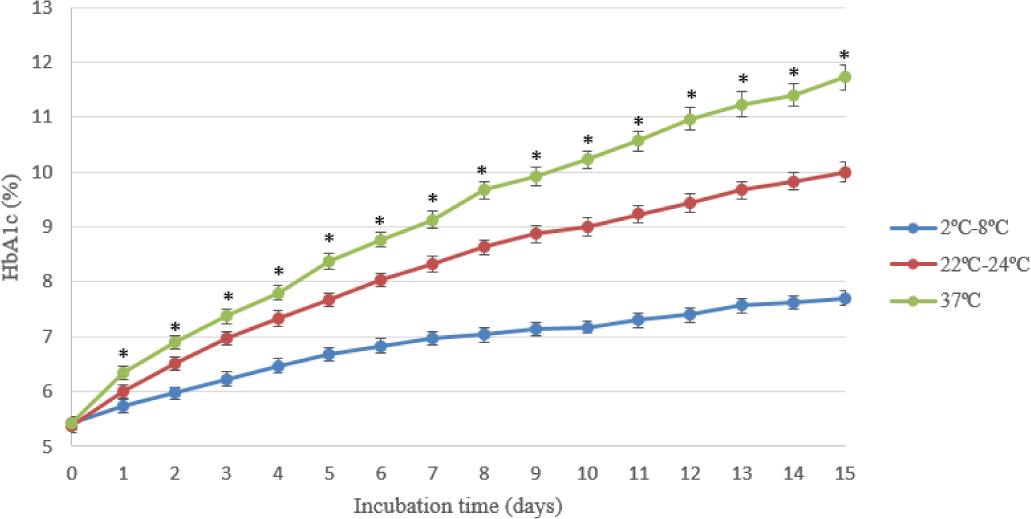
As shown in Table 2, the rate of increasing concentration of HbA1c increased continuously to the fifteenth day. The level of HbA1c at 37°C was the highest (11.73±0.23%) and the lowest at 2-8°C (7.7±0.13%) on the last day.
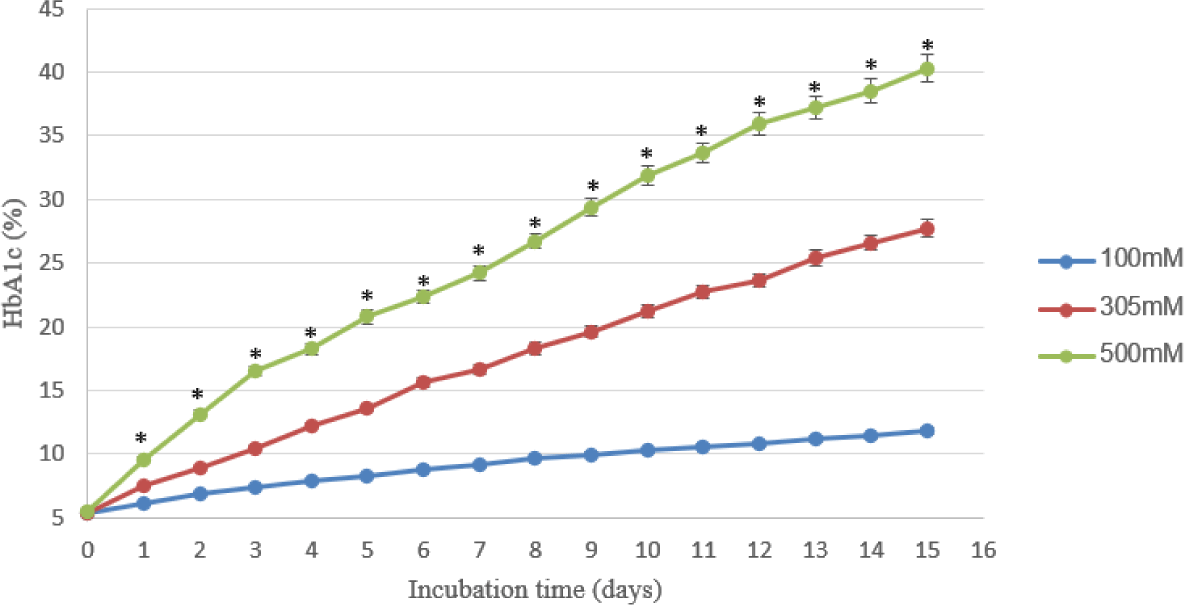
In phase 3, RBCs were incubated in BAGPM solutions with different glucose concentrations at 37°C. As shown in Table 3, the concentration of HbA1c in the samples at three levels of glucose increased dramatically with the highest at 500 mM glucose (40.3±1.05%) on the fifteenth day.
After the above experiments, the HbA1c levels at pre-diabetes (6.0±0.12%) and diabetes (9.6±0.17%) were observed by incubating RBCs in BAGPM solution with 100 mM (initial HbA1c concentration 5.37±0.12%) and 500 mM (initial HbA1c concentration 5.47±0.11%) in 24 hours at 22°C - 24°C and at 37°C.
4. DISCUSSION
The HbA1c concentrations at medical decision levels of samples are an important criterion in EQA programs to assess the competence of participants. Producing HbA1c’s EQA control materials set that covers all medical decision levels include normal range (4% - 5.7%), prediabetes (5.7% - 6.4%), and diabetes (≥ 6.5%) is necessary to ensure the quality of participants [1]. In donor blood that was kept in the refrigerator at 2°C to 8°C in the buffer solution containing about 27 mM glucose, the HbA1c concentration was significantly higher in RBC units after 42 days of storage without clinical relevance [9-9]. In Table 1, the mean initial HbA1c concentration of the sample in incubated solutions is from 5.40±0.13% to 5.47±0.12% in the normal range that is suitable [12]. The results showed that there was an increase of HbA1c concentrations in the Ringer, BAGPM, Saline, and PBS solution during the 15 days at 37°C. In figure 1, the percentage of HbA1c in BAGP-M and Saline get a higher growing trend line than in PBS and Ringer to the fifteenth day with increasing 11.57±0.2% and 11.07±0.18% respectively. When incubating RBCs at 4°C, Mortensen et al did not find any change in HbA1c concentration in sixty hours in Saline with 25 mM glucose, with higher glucose concentration (100mM glucose) in this study, the mean of HbA1c level in Saline buffer increased from 5.47±0.12% to 7.00±0.14% in 3 days [13]. The hemolysis visually occurred from day 6 and especially in the Ringer solution. So, the BAGPM solution was chosen to incubate RBCs to increase HbA1c concentration in the next experiment.
In the experiment on HbA1c concentrations over 15 days at Glucose 100 mM in BAGPM solution about the effect of temperature (Table 2), the trend line at every temperature increased significantly with the highest peak (11.73±0.23%) on the fifteenth day at 37°C. At the same temperature, Spicer et al found that the concentration of HbA1c increased about 17% on day 15 in Ringer solution with 250 mM glucose [7], the higher glucose concentration may cause the increasing HbA1c concentration. On the other hand, the changing of HbA1c level was beyond the clinical signs in the study of Prosenz et al under standard blood banking conditions (approximately 27 mM glucose) after day 42. The increase nearly the half in this study at 2°C-8°C maybe because of glucose concentration higher than 4 folds the Prosenz et al study [9].
In Table 3, with the same temperature (37°C), the rate of increasing HbA1c level depends on the concentration of glucose. The HbA1c concentrations were continuously raised to day 15 demonstrating that this is an irreversible reaction. In the study by Smith et al, the rate of HbA1c formation of RBCs incubated in Ringer 55 mM glucose with 10% bovine albumin at 37°C was 0.4% per 24 hours and linearity to the eighth day [14]. At the same time, the results also showed that the concentration of HbA1c in the red blood cell samples incubated in BAGPM’s solution with 3 different levels of glucose also showed that the increase in the maximum HbA1c concentration depends on the glucose concentration in the solution. This result is similar to the study of Yuliya V. Kucherenko in which the glycated process was accomplished by exposure to high glucose concentrations (40 and 100 mM) [4].
In our study, the hemolysis visually occurred from day sixth in all experiments, the higher the temperature and the glucose concentration, the faster the hemolysis occurred. In the Spicer experiments, the hemolysis occurs from day 4 and increases to day 21[7]. To minimize the hemolysis in the samples, and make good external quality control materials, the time after 24 hours were chosen. There were statistical differences between 100, 304, and 500 mM glucose, so RBCs were incubated in BAGPM buffer solution 100 mM glucose to produce prediabetes EQA materials. For the HbA1c concentration inside the diabetes range, the RBCs were incubated at 37°C for 1 day in the solution containing 500 mM glucose [1].
Conclusion
We have identified appropriate conditions to prepare HbA1c standards for prediabetic and diabetic levels. We recommend that standards for HbA1c concentrations be prepared by incubating RBCs from non-diabetic donor blood in BAGPM solution containing glucose at 37°C for 24 hours. Glucose concentrations should be 100 mM and 500 mM, respectively, for prediabetic level (HbA1c ~ 6.0 ± 0.12%) and diabetic level (HbA1c ~ 9.6 ± 0.17%).









