1. INTRODUCTION
Total anomalous pulmonary venous connection (TAPVC) is a rare condition, accounted for 1,5-3% of cyanosis congenital heart diseases [1]. Without surgery, the 1-year mortality were >80% [2, 3]. Due to evolution of perioperative care and surgical technique, mortality of TAPVC has improved dramatically in the the past decades. There are four subtypes of TAPVC [4]. Of those, supracardiac and intracardiac types are more common [5]. Meanwhile, infracardiac and mixed TAPVC have a poorer prognosis due to the risk of postoperative pulmonary venous stenosis (PVS) [2, 6, 7]. This complication which is well-known as a high-risk of mortality and morbidity often occurs within first 6 to 12 months after complete repair [8]. Sutureless technique, which were performed by suturing the left atrial wall with pericardium of pulmonary venous confluence (PVC), were initially applied to repair these complications. However, in the efforts to prevent postoperative PVS, sutureless repair has been currently adopted for primary TAPVC in many cardiac worldwide centres. A number of reports demontrated a favourable outcomes of this novel technique for TAPVC in terms of postoperative PVS rate and reoperation rate for this complication [9, 10]. In the light of these results, our institution have gradually applied sutureless repair for primary TAPVC since 2018 for several infracardiac cases. From mid-2019, we have expanded the indications for other subtype of TAPVC and conducted a prospective study to assess the outcomes of this novel technique, simultaneously. The study will be concluded at the mid of 2023. Notwithstanding, this paper’s purpose is to assess the early outcomes of sutureless technique after 18 months of recruitment of prospective study with respect to mortality including operative and overall, postoperative PVS and technical aspects.
2. MATERIALS AND METHOD
A prospective observational study was conducted at Children’s Hospital 1, Ho Chi Minh City, Vietnam from June 2019. Our current indication for sutureless closure, which was similar to LoRito, consists of all TAPVC cases of supra cardiac, infracardiac, mixed types and intracardiac types with PVS [11]. We excluded TAPVC with single ventricle physiology, atrial isomerism or heterotaxy syndrome. During June 2019 to December 2020, there were 36 cases admitted to our hospital. Four cases died prior to surgery. Eleven intracardiac TAPVC without PVS were repaired with conventional surgery, including unroofing coronary sinus and closing the atrial septal defect with a pericardial patch. Four infracardiac TAPVC which met the indication of sutureless underwent conventional repair because of surgeons’ preference, at the early experience of the sutureless technique. Subsequently, seventeen TAPVC cases which were repaired with sutureless closure were enrolled in this study. Of those, 14 (82.4%) cases were supracardiac type and 3 (17.6%) cases were infracardiac type. Our primary outcome was mortality and postoperative PVS. Patients’ demographics are shown in Table 1.
Majority of TAPVC cases were diagnosed with echocardiography. Computerized tomography were indicated when required for diagnosis confirmation.
After induction of general anesthesia, cardiopulmonary bypass (CPB) was initiated with bicaval cannulas and one arterial cannula via full midline sternotomy. Cardioplegia was instilled via aortic root. Moderate hypothermia with the continuous flow was used.
- Supracardiac and infracardiac type: the anastomosis was performed with retrocardiac approach as Mavroudis described in 2001 [12]. The heart was retracted to the right and superiorly out of the pericardial cavity to expose PVC and the vertical vein. The vertical vein was looped. The incision was made on the left atrium and the confluence. In the left atrium, the incision was larger and reached the atrial septal defect. In the confluence, the incision went upstream to each pulmonary vein. The side-to-side anastomosis was performed with 7.0 sutures between the pericardium surrounding pulmonary veins with the left atrial wall (Figure 1). Then, the atrial septal defect was closed directly, and a small 3mm patent foramen ovale was left. After coming off bypass, pulmonary arterial pressure was routinely measured and compared with systemic pressure. If pulmonary pressure was >2/3 systemic pressure, pulmonary arterial (PA) line was set-up to instil ilomedine. Delayed chest closure was performed if hemodynamic status was unstable after weaning from bypass.
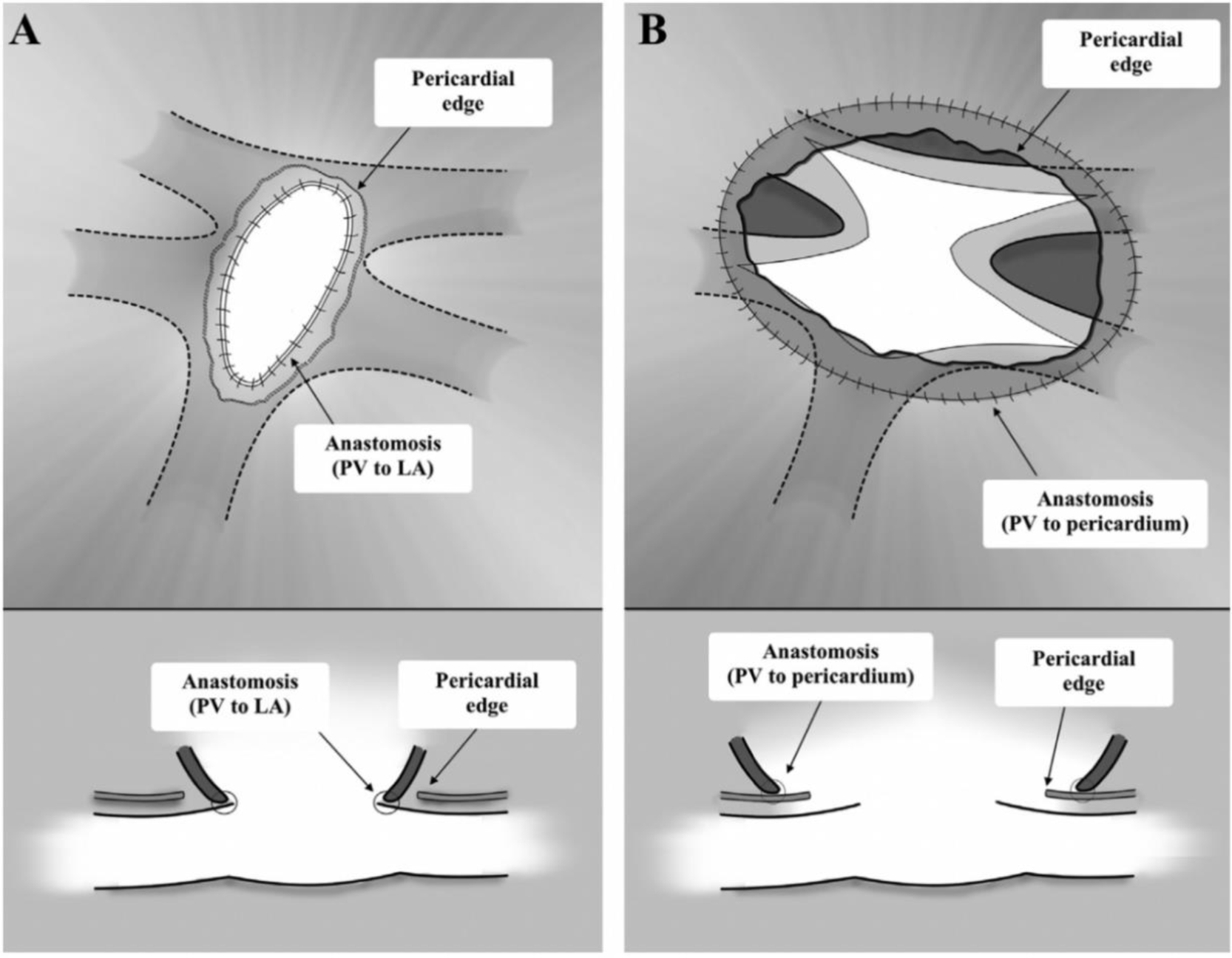
- Intracardiac TAPVC type was repaired by unroofing coronary sinus and closing the atrial septal defect with a pericardial patch. Sutureless technique was applied when the confluence connected with coronary sinus or right atrium through a narrowing vein, or individual pulmonary veins were small intraoperatively. Incision was made into the stenosed parts and correspoding left atrium. The anastomosis was created with 7.0 sutures between the pericardium around stenosis and left atrium. All other parts of the operation was proceeded similar to other TAPVC subtypes.
- Mixed TAPVC: combined these two techniques.
All TAPVC cases were performed by one of two experienced surgeons. All postoperative echocardiography was cut by a single experienced cardiologist. The researcher just observed the cases and assisted with the surgery. The researcher did not involve in deciding the choice of operation and time of surgery.
Data was obtained from medical records and the hospital information system. Pre-operative clinical information included age at admission (days), weight at repair (kilograms), subtype of TAPVC, and pulmonary venous obstruction (PVO). Associated congenital defects were defined as deformities of organs including cardiac and non-cardiac. Patent ductus arteriosus and atrial septal defect was considered as common features of TAPVC. Preoperative PVO was identifined when peak velocity through each pulmonary vein on pre-operative echocardiography >1.8m/s or intraoperative findings of obstruction. Patients who had diagnosis of obstructed TAPVC underwent emergent surgery. Surgical data comprised of type of surgery (defined as emergent – within 24 hour of admission for TAPVC repair; urgent – between 24-48 hour; and elective – after 48 hour), closure technique (sutureless), operation duration in minutes (including aortic clamping time and CPB time). Reoperation was defined as a surgery performed to repair postoperative obstruction after patients were transferred to CICU. Redo anastomosis was defined as a surgery performed before patients were transferred to CICU. Post-operative surgical information consists of arrythmias, ventilatory time (number of days from surgery to extubate), length of cardiac intensive care unit (CICU) stays (number of days from the date admitted to CICU to the date transfered to cardiac surgery department), length of hospital stay. Diaphragmatic palsy was confirmed with ultrasonography or fluoroscopy after hemi diaphragm was found to be elevated on chest xrays. Chylothorax was diagnosed with confirmation of cholesterol and triglycerides in pleural fluid.
Operative mortality was defined as death occured within 30 days of operation or at the same admission of primary surgery. Postoperative pulmonary venous stenosis was identified based on pulmonary venous score system which was introduced by Yun [13]. In Yun’s method, mean gradient pressure through anastomosis and all four pulmonary veins were measured. This following score was correspoding for one pulmonary vein. Zero point if mean gradient < 2 mm Hg; 1 point if mild stenosis (mean gradient 2.0–6.9 mm Hg); 2 points if severe stenosis (mean gradient >7 mm Hg); and 3 points if complete occlusion (no flow). Then, the sum of the individual pulmonary vein scores is calculated with minimum score of 0 (no stenosis) and maximum score of 12 (all four pulmonary veins). This score system had not been measured preoperatively because of diversity of cardiologists who performed the TAPVC diagnosed echocardiography and time-consuming which was not approriated for critical ill neonates and infants.
Data were described in medians with ranges, or means ± standard deviation for quatitative variables and frequencies, percentage for quanlitative variables. SPSS 26.0 software was used to analyze the data.
This study was approved by the Children’s Hospital 1 Committee Ethical Board (number 13/GCN-BVNĐ1) and was done in compliance with the principles of Declaration of Helsinki. We got individual written informed consent for all patients enrolled on this study. The consents were signed by one of two parents or guardian of children in case of no parents.
3. RESULTS
There were 17 cases, including 12 male and 5 female, with a median age at the admission of 44 days (range 3-1010), median weight at repair of 3.9 kg (range 2.4-11). Only 3 (17.6%) cases were premature. Cyanosis and tachypnea were the most common initial presentations with 47.1% (8/17) and 35.3% (6/17), respectively. Three (17.7%) cases needed cardiopulmonary resuscitation (CPR) pre-operatively. Majority TAPVC cases in our series were diagnosed with echocardiography; 5 cases required computed tomography angiogram because of unclear diagnosis in echocardiography. There was no mixed case or intracardiac case with PVS in our series. Of 17 TAPVC cases, 14 cases were supracardiac type (14/17, 82,4%) with obstructed TAPVC in over half (8/14, 57.1%) and 3 infracardiac cases with obstruction in 2/3 (66.7%). Afterward, 9/10 obstructed TAPVC cases were operated emergently; the other obstructed case was urgent surgery; the remainder were elective surgeries. The supracardiac obstructed TAPVC case which operated urgently underwent balloon atrial septostomy procedure pre-operatively due to presenting obstruction at atrial level. The demographic characteristics and pre-operative information were summarized in Table 1.
As shown in Table 2, 9 (52.9%) patients developed arrhythmia during CICU course and resolved before discharge. These were sinus node dysfunction (5/17, 31.3%), ectopic junctional tachycardia (3/17, 18.8%), supraventricular tachycardia (1/17, 6.3%). Four patients presented intraoperative ventricular fibrillation which were treated with immediately cardioversion. Fortunately, there was no other ventricular arrhythmia during in-hospital postoperative course and follow-up. Our median aortic clamp time was 66 minutes (32-138), and our median bypass time was 112 minutes (range 86-212). There was no intraoperative pulmonary venous stenosis or atresia in our series. Median postoperative bleeding in the first 6 hours was 1.1 ml/kg/h (range 0.3-4.2). Delayed chest closure was performed in 6 (35.3%) patients. PA line was placed in 4 (23.5%) patients because pulmonary pressure was >2/3 systemic pressure. Mean ventilatory time was 3.6±0.5 days. Median CICU time was 6.5 (range 1-20) days. The median length of stay (LOS) was 14.5 (range 8-39) days. In-hospital mortality was 5.9% (1/17). This case was a premature three days-old male neonate transferred from another hospital. He was on high-frequency oscillatory ventilation upon arrival. He was operated right after definitive diagnosis of supracardiac obstructed TAPVC and died at postoperative day 5 with multiple organ dysfunction syndromes which had already presented from the pre-operative period. Except for this cases, the remainder are survivors. After mean follow-up time of 12.3±6.4 months of 16 TAPVC cases (minimum of 4 and maximum of 24 months), there was no pulmonary venous stenosis with median pulmonary venous score of 0 (range 0-1).
4. DISCUSSION
Since 2018 sutureless closure had been adopted at Children’s Hospital 1, HCMC, Vietnam for a few infracardiac TAPVC cases with good results. With the encourage of initial pilot, we expanded the indication for other subtypes since mid-2019. During follow-up time of at least 4 months, early mortality was 5.9% (only 1 case) and no postoperative PVS was recorded. This was an primarily acceptable results after application of new technique for tough defects as TAPVC in developing country like Vietnam.
During 2018-2019, both techniques was used simultaneously. Hence, in the early-stage to switch from conventional repair to sutureless closure, there were four infracardiac TAPVC underwent the traditional technique because our surgeons were concerned of postoperative bleeding due to the thin pericardium in neonates, especially in premature or low operative weight (<3kg) patients. Eventhough, Sakamoto and Horner suggested that low-weight at repair might have benefited from sutureless closure due to reducing the risk of postoperative pulmonary venous obstruction, bleeding is also major concern of the technique in this group of patients [4, 14]. Usually, this complication was encounted due to incision extending to pulmonary venous orifices which may lead to detach the hilar pleuropericardium junction or due to adhesion-free thin posterior pericardium, especially in neonates. However, this complication was avoidable with careful manipulation of the thin pericardium within and after performing atrio-pericardial anastomosis. Likewise, an incision of a few milimeters from two corners of PVC into each pulmonary vein will be sufficent for anastomosis. A deeper incision into individual pulmonary vein is unneccesary. In our earlier era of the sutureless technique, two TAPVC cases which had intraoperatively disastrous hemorraghe required redo the anastomoses. Rupture of suture line and breach of left pleuropericardial approximate were the leading causes in these cases.
From our initial experience, sutureless technique was more straighforward than conventional repair. For the reason that, suture line in sutureless closure was located on posterior pericardium. In the like manner, the potential anastomotic torsion between two structures that belonged to different planes (left atrium and posterior pericardium) was also diminished in comparision of conventional repair. By the same token, for those infracardiac TAPVC case with intricate patterns like Y, T or tree shapes, sutureless anastomosis was effortlessly achieved in our series thank to suturing pericardium instead of PVC which was often narrower, deeper and subsequently harder to sew than pericardium. Additionally, the anastomosis in neonates seemed to be easier than in infants. Operative field in neonates which was smaller but more superficial than in infants facilitated for anastomotic performance. In contrast, a larger but deeper surgical field in infants caused more time-consuming anastomosis.
One critical issues in open-cardiac operation is bloodless or less-blood operative field for repairing defects. Some authors advocated deep thermia and cardiac arrest (DHCA) to achieve this purpose but not all. DHCA was proved to be a risk factor for mortality and morbidity related to neurology development [8, 14]. Therefore, most centers recently had abandoned this approach and have been using moderate or mild hypothermia with the continuous flow [15-17]. In our Children’s Hospital 1, HCMC, Vietnam, DHCA was applied for only two TAPVC cases during 2008-2010 including one mixed and one infracardiac case. Since then, all other TAPVC cases underwent repair with continuous flow. To perform atrio-pericardial anastomosis with ease, bloodless field was managed with 3 pump suckers. Of those, each rigid sucker was placed into one-sided pulmonary veins either upper or lower, one soft sucker was situated through patent foramen ovale.
In our series, there was only one in-hospital death. This 3-day-old newborn developed profound hypoxia and respiratory failure. He was intubated and administrated high frequency oscillatory (HFO) ventilator due to rapid deterioration of hemodynamic status but there was no clinical improvement. He was transferred to our Children’s Hospital 1 after diagnosis of supracardiac obstructed TAPVC on his 4th day of life. Upon arrival, his peripheral saturation was approximate 40% on HFO and had symptomes of renal and liver failure. Operation was performed emergently on the day of admission. Eventhough bypass was successfully weaned off, his postoperative status was fragile with relentless end-organ failures, pulmonary edema, and pulmonary hypertension. Subsequently, he died on postoperative day 5 without clinical response. In litterature review, for such cases, some worldwide centers applied stenting vertical veins to temporarily resolve obstruction or employed extracorporeal membrane oxygenation (ECMO) as palliative treatment for impaired cardiac function or injuired lungs [18, 19]. Nitric oxide is well recognised as treatment for severe pulmonary hypertension [20]. Unfortunately, ECMO was not accessible for neonates at our hospital at that time, stenting vertical vein have not been performed in any patients because of high-skilled requirement and nitric oxide has not been available so far. Because of limited available resources, HFO was our only choice for the neonate and it was ineffective for this situation. Meadows et al presented a 1-day old infracardiac TAPVC case with homogeneous clinical scenario [21]. This case underwent ECMO administration and descending vertical vein and ductuc venosus stenting prior to surgery. Subsequently, he underwent prosperous TAPVC repair on the his 8th day of life with sutureless technique after successfully weaning off ECMO. However, this hybrid approach which required high-skilled multidisciplines overcome the ability of most of cardiac teams in developing country like us.
Technically, although left diaphramatic palsy was another concern of sutureless repair, there was no such case in our series. Notwithstanding, postoperative chylothorax was represented in two supracardiac TAPVC cases as shown in Table 2. These two cases was treated with conservative mangement including lipid-limited partial parenteral nutrition. This condition at both cases was resolved before discharge. Peritoneal dialysis was used to support renal function due to acute renal failure after heart surgery which was common in neonates [22]. In our series, four obstructed TAPVC neonatal cases which required postoperative peritoneal dialysis for within first two days were withdrawn these catheters due to good urine output.
As shown in Table 3, our aortic clamping time and bypass time were slightly longer than Lo Rito, Yamagawa and Shi et al. [9, 11, 23]. These authors used to apply DHCA in their earlier era of performing sutureless technique. Moreover, the preoperative PVO rate in our series was higher than the other three studies. These may partially explain for the longer bypass time in our series. In comparison of aortic clamp time, our result was similar to LoRito and Yamagawa but longer than Shi. For the reason that, there was no intracardiac TAPVC with PVS case in our series which usually had a shorter operative time than other subtypes due to simpler procedure. Furthermore, our experience of 18 month was still barely in comparison of those centers, therefore, our longer operative time was obvious. Eventhough our operative and overall mortality were acceptable, the period of follow-up in our series was much shorter than other authors. We keep following up these patients.
| Study | Aortic clamp time (mins) | Bypass time (mins) | Operative mortality n(%) | Overall mortality n(%) | Follow-up time |
|---|---|---|---|---|---|
| Our study (N=17)* | 66 (32-138) | 112 (86-212) | 1 (5.9%) | 1 (5.9%) | 12.3 months |
| Lo Rito [11] (N=69)* | 58 (47-73) | 84 (74-107) | 1 (1.4%) | 7 (10.2%) | 6.4 years |
| Yamagawa [9] (N=21)** | 62.3±19.3 | 93.8±23.2 | - | 4 (19%) | 21.1 months |
| Shi [23] (N=78)** | 48.5±19.4 | 94.6±40.6 | - | 5 (6.4%) | - |
The limitations of our study were small sample size and short-time follow-up. As well as, there was no available conventional repair data of similar end-point outcomes in corresponding follow-up time so as to compare with. Our experience with this technique is still minor. However, this paper has value in terms of helping limited-resource hospitals, centres, or institutions that have not applied this technique for their patients and now would like to consider. After this primarily encouraging results, sutureless technique is going to be a standard of operation for TAPVC at Children’s Hospital 1, HCMC, Vietnam. We are continuing to follow up these patients and contining to recruit.








