1. INTRODUCTION
Pseudomembranous colitis refers to an acute inflammatory condition of the colon which is characterized by elevated yellow-white plaques that coalesce to form pseudomembranes on the colonic mucosa [1]. In recent decades, PMC emerged as a common complication of antibiotic use, and almost all cases are now caused by infection with toxin-producing strains of Clostridium difficile [2]. Patients with the condition commonly present with abdominal pain, diarrhea, mucus in stool, and fever. Ascites, however, is not considered a well-known feature of PMC.
Here, we report a rare case of PMC presenting with massive ascites and describe the characteristics of the ascitic fluid specimen.
2. CASE REPORT
A 52-year-old female presented to our hospital with a 5-day history of frequent bloody, mucoid, jelly-like diarrhea (10 times per day), abdominal cramps, and new abdominal distention. She was treated at local hospital with broad-spectrum antibiotics (imipenem and ciprofloxacin for 4 days) without any improvement. The patient was a housewife. She lived in Khanh Hoa, a province of Vietnam, and did not travel away from the province before her symptom onset. She had no remarkable past medical history except for a 7-day use of amoxicillin/clavulanate per oral for her sinusitis a week before her symptom onset. She did not contact anyone who had the same symptoms.
At admission, the patient was alerted and oriented, blood pressure was 130/80 mmHg, temperature was 370C, heart rate was 84 beats per minute, and respiration rate was 20 breaths per minute. There was no icterus, spider nevus, or palmar erythema. The base of the right lung was dull on percussion with decreased tactile fremitus and decreased breath sound. Physical examination of the abdomen revealed abdominal distention with tenderness on palpation and dullness on percussion. There was bilateral pedal edema. The remainder of clinical examination was unremarkable.
Her complete blood count showed leukocytosis (white blood cell count of 15.9 x 109/L with 79 % neutrophil). Biochemical tests showed the following information: CRP 76.1 mg/L (normal range < 6), albumin 2.5 g/dL (normal range 3.5 - 5.5), potassium 2.5 mmol/L (normal range 3.5 – 5.5). Aminotransferase, bilirubin, prothrombin time, blood urea nitrogen, serum creatinine were within normal limit. Urine protein to creatinine ratio was 420 (mg/g). Nephrotic syndrome was ruled out and the abnormal protein to creatinine ratio was supposed to be caused by acute colitis. Stool specimen was positive for erythrocytes and leukocytes but without evidence of ova and parasite. Stool culture for common enteric pathogens was negative. Serology tests were negative for Entamoeba and Strongyloides.
Chest X ray showed right pleural effusion. CT imaging confirmed the presence of ascites but also showed diffuse wall thickening of the entire colon (Figure 1). Echocardiography showed normal left and right heart function with no heart valve disease.
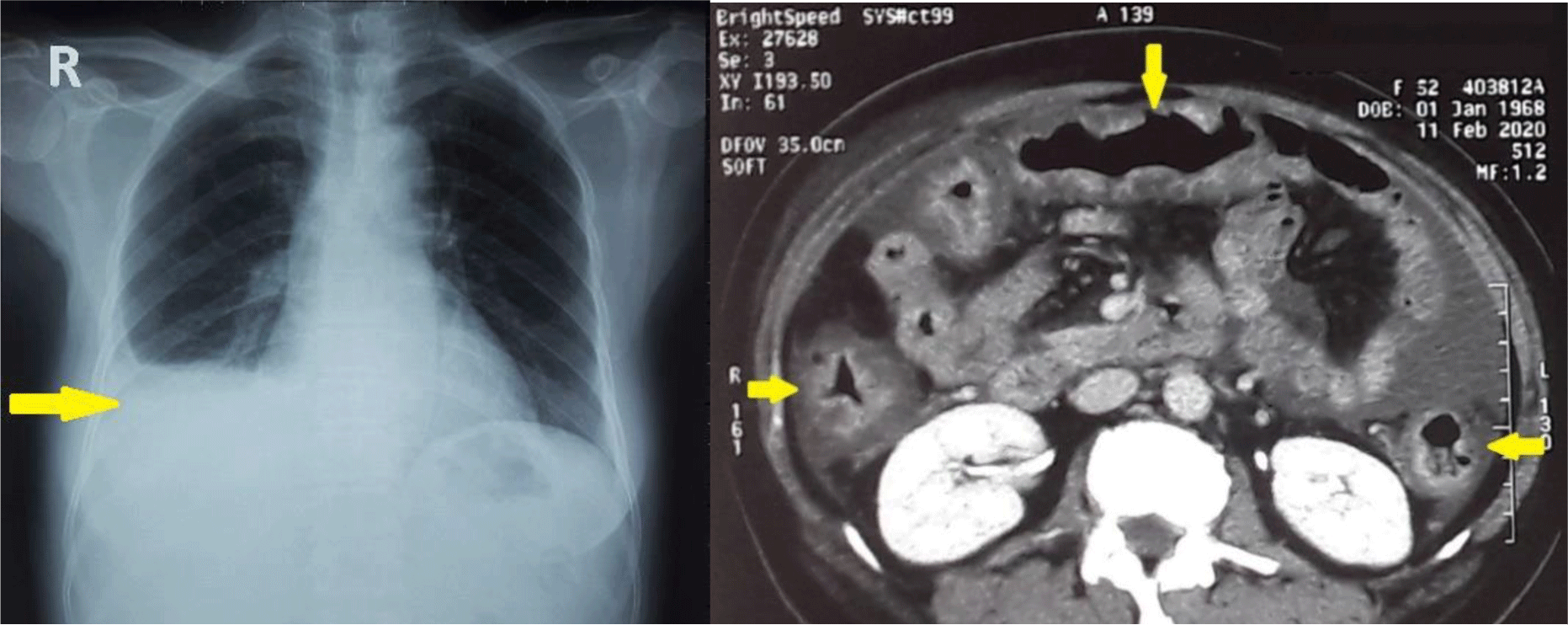
Abdominal paracentesis was performed. The fluid was cloudy and contained 2599 leukocytes/mm3, most of them polymorphonuclear (75%). The SAAG was low (0.8 g/dL). The ascitic fluid protein was 2.6 g/dL, adenosine deaminase was 9.3 U/L (normal range 4 – 24). Culture and fluid gram stain were negative. Adenosine deaminase (ADA) was 9.3 U/L (normal range 4 – 24) and Mycobacterium tuberculosis PCR was negative which ruled out tuberculous ascites.
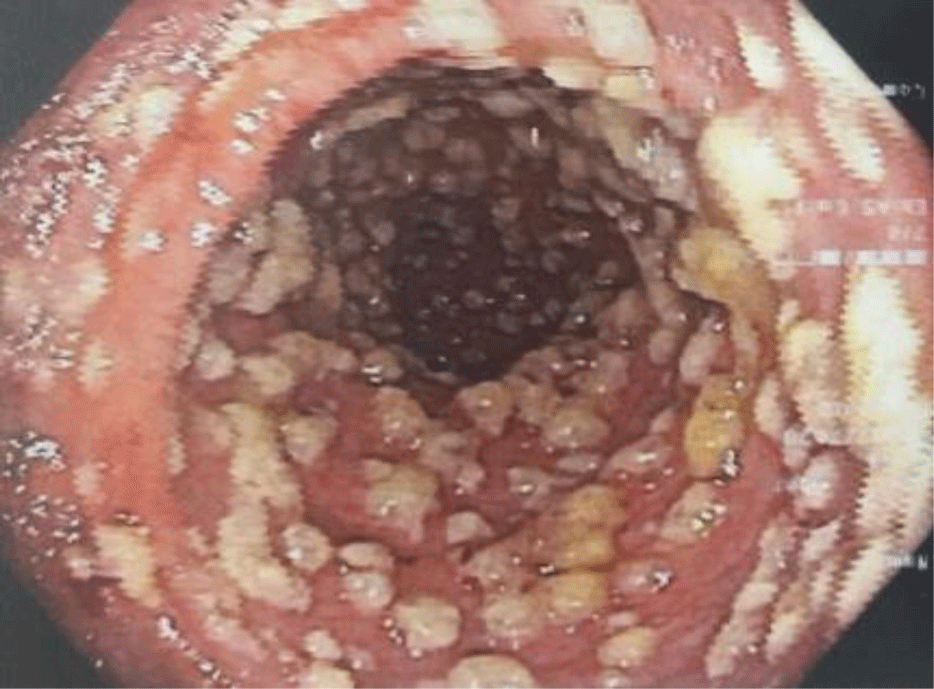
The patient was treated empirically with a combination of ciprofloxacin IV 400 mg q12h and metronidazole IV 500 mg q8h and was scheduled for flexible sigmoidoscopy. On the following day, the patient underwent flexible sigmoidoscopy which showed ovoid yellow plaques of 2 - 10 mm in diameter separated by areas of hyperemic mucosa (Figure 2), suggestive of PMC. No biopsy was performed. Stool specimen was collected again testing for Clostridium difficile. The Clostridium difficile Real Time PCR test came back negative for toxigenic strains of Clostridium difficile. A repeat PCR test was ordered 7 days after the first one, however, the result was also negative.
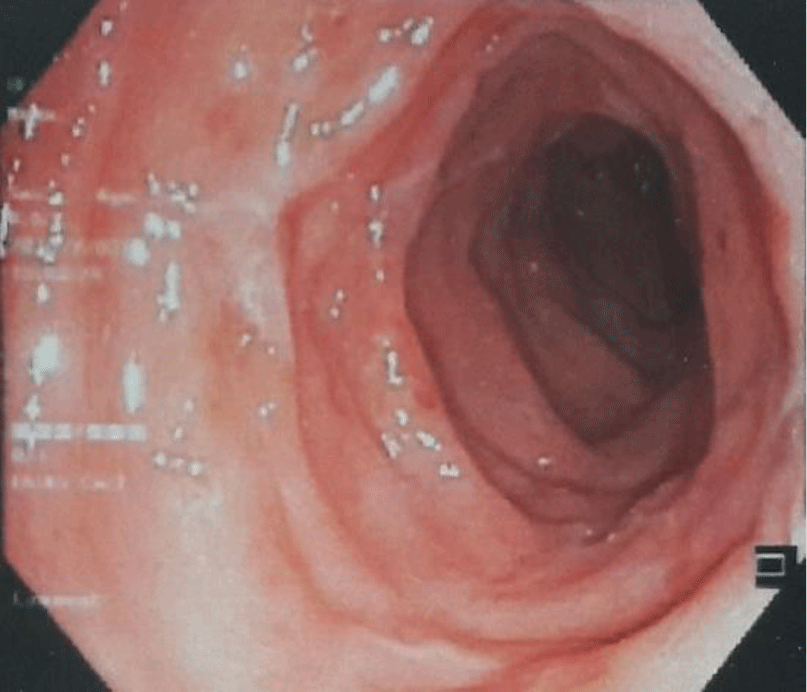
Ciprofloxacin was discontinued and vancomycin 250 mg PO QID was started. After several days, her diarrhea subsided (1 – 2 bowel movements a day with no blood and mucus). After 8 days of treatment, no ascites was found by ultrasound. Flexible sigmoidoscopy showed healing of rectal mucosa with disappearance of pseudomembrane (Figure 3). The patient was discharged after 14 days of treatment. The patient had an uneventful follow-up at local hospital 3 months after discharge.
Clostridium difficile Real Time PCR assay was performed using the Sacace Biotechnologies Clostridium difficile + ToxA + ToxB Real-TM kit according to manufacturer’s instructions. The assay was used for the qualitative detection of Clostridium difficile and differentiation of genes encoding toxins in stool. The kit allows detecting Clostridium difficile and tox genes DNA in 100% of the tests with a sensitivity not less than 1000 copies/ml. The specificity of the kit was 100%.
3. DISCUSSION
PMC is an acute, exudative inflammation of the inner lining of the colon. The colonical mucosa is often covered with loosely adherent nodular or diffuse exudates in severe cases. The coalescence of these plaques forms an endoscopic appearance of yellowish pseudomembranes along the colorectal mucosa. PMC is usually associated with antibiotic use, which may disrupt the ecology of the normal intestinal microbiota [3]. Almost all antibiotics can induce PMC, but clindamycin, cephalosporins, and fluoroquinolones are the most frequently cited [4]. Our patient was prescribed amoxicillin/clavulanate a week before her symptom onset.
PMC results most commonly from CDI [1]. Clostridium difficile is a spore-forming, gram-positive bacillus. It is a well-recognized cause of antibiotic-associated diarrhea. The spectrum of clinical manifestation of CDI may include, in increasing order of severity, absence of symptoms, antibiotic-associated colitis without the formation of pseudomembranes, and PMC. PMC is a severe but rare complication of the infection, related to the bacteria production of enterotoxin A and B [5]. Clostridium difficile was the most suspected cause of PMC in our patient. Various tests could be used to diagnose CDI including enzyme immunoassays (EIAs) to detect toxin antigens in stool, nucleic acid amplification tests (NAAT) such as PCR, and the tissue culture cytotoxicity assay (gold standard). EIAs are easy to perform and have high specificity (83-98%). However, they are less sensitive (75-95%) than the cytotoxicity test. PCR assays have high sensitivities and specificities of over 90% compared with toxinogenic culture or cytotoxicity assays and are superior compared with toxin EIA [2]. In our hospital, PCR assay was the only test available to diagnose CDI. In this case, the Clostridium difficile Real Time PCR test, which was performed twice, was negative. There are 2 possible explanations for PMC with negative Clostridium difficile PCR tests. Firstly, PMC could result from etiologies other than CDI such as ischemic colitis, collagenous colitis, inflammatory bowel disease, viral infection with cytomegalovirus (CMV), other bacterial and parasitic organisms, and multiple drugs and toxins [1]. However, PMC caused by these etiologies will not improve with metronidazole and vancomycin. Patients with no treatment response warrant further evaluation for other causes. The second possible explanation for the negative PCR test is that it was a false negative result which may have been caused by the effect of therapy. The stool was obtained 2 days after the patient was placed on metronidazole as empirical treatment. As described by Sunkesula et al., for PCR, 14%, 35%, and 45% of positive CDI tests converted to negative after 1, 2, and 3 days of treatment, respectively [6]. A study conducted by Robert F. Luo et al. also showed that repeating Clostridium difficile tcdB PCR within 14 days of a negative result yields little relevant clinical data, other than confirming the negative result from the initial test, in a considerably majority (97.5%) of tests [7]. Therefore, in this case, a repeat test is not necessary.
Watery diarrhea with mucus or occult blood, anorexia, nausea, vomiting, low-grade fever, and lower abdominal pain are the most common symptoms of PMC. Ascites, however, is not a well-known feature of PMC. This patient presented with massive neutrocytic ascites and a low SAAG, indicating an exudate process. Eli Zuckerman et al. performed paracentesis on 5 patients with PMC, all of whom presented with low SAAG and neutrocytic ascites. In 4 out of 5 cases, ascites disappearance coincided with the resolution of PMC as in our case. Three out of 5 patients (60%) reported by Zuckerman had acquired immunodeficiency syndrome [8]. In addition to antibiotic therapy, advanced age, duration of hospitalization, chemotherapy for malignancy, HIV infection, and IBD are risk factors of CDI [9]. This patient would need to have a full colonoscopy in the future to rule out IBD as well as get tested for HIV infection. George I. Tsourous reported a case of PMC presented with massive ascites, the same ascitic fluid characteristic was found [10]. There are several differential diagnoses of low-SAAG ascites, such as malignant conditions, serositis accompanying connective tissue disease, chronic granulomatous or infectious peritonitis, pancreatic, biliary, or nephrogenic ascites, or hypoalbuminemia. In our case, ascites was the complication of PMC. Three potential mechanisms have been suggested for the ascites formation in PMC, including hypoalbuminemia, toxin-mediated generation of cytokines that enhance vascular permeability and transmural colonic inflammation with microperforation and infectious peritonitis [8]. In our patient, hypoalbuminemia may contribute to the formation of ascites as the patient presented with generalized edema (ascites accompanied with bilateral pedal edema and pleural effusion). However, hypoalbuminemia did not explain the high protein and exudative features of the ascitic fluid. Transmural colonic inflammation with microperforation and infectious peritonitis hypothesis was not supported by the negative gram stain and bacterial culture of ascitic fluid. However, we could not rule out this possibility since this patient was treated empirically with broad-spectrum antibiotics before admission. A study showed that C. difficile toxin induced generation of inflammatory cytokines, which may in turn enhance vascular permeability [11]. This hypothesis was attractive but we were unable to confirm this possibility.
Our patient had severe CDI as she had leukocytosis with white blood cell count of > 15000/ mm3 and flexible sigmoidoscopy showed PMC [5, 9]. Therefore, a regimen with a combination of metronidazole IV and vancomycin PO for 10 – 14 days was chosen [2, 9]. The patient showed a marked response with no ascites found on ultrasound as well as healing of the colon mucosa.
Conclusion
In conclusion, we reported a rare case of PMC presenting with massive ascites and described the characteristics of the ascitic fluid. In patients with PMC, cross-sectional imaging and further work-up are warranted to look for intra-abdominal process and even neutrocytic ascites, which can be a complication of this condition. It is also important to be aware that empirical therapy in patients with suspected CDI may cause false-negative test results, which may lead to the progression of illness if the diagnosis is missed.








