1. INTRODUCTION
Pulmonary manifestations of systemic lupus erythematosus (SLE) are diverse, including airway disease, pulmonary infection, pleural effusion, pleuritis, alveolar hemorrhage, interstitial lung disease, acute lupus pneumonitis, thromboembolic disease, and pulmonary artery hypertension.
In a cross-sectional study of 283 patients with SLE, pulmonary hypertension (PAH) was present in 12 patients (4 percent) based on Doppler echocardiography [1], and severe pulmonary hypertension was noted in about 1 percent. The severity of PAH is estimated by systolic pulmonary artery pressure (sPAP) index through transthoracic echocardiography (TTE). Severe PAH is defined as sPAP > 40 mmHg.
PAH in SLE becomes especially noticeable in pregnant women due to an increase in lupus activities during pregnancy [2]. Pathological mechanisms have been documented that pregnancy promotes PAH, perhaps by hormonal shifts required to maintain pregnancy. The process can lead to acute right ventricular failure [2]. Therefore, SLE-associated-PAH in pregnant women poses a higher maternal and fetal risk compared with pregnancy in healthy women.
We herein reported a case of a pregnant woman who had severe SLE-associated-PAH. In this case, pulmonary artery hypertension was considered as an initial manifestation of SLE. The patient’s condition was stabilized by immunomodulator and vasodilator therapy; however, gestation had to be terminated.
2. CASE REPORT
A 33-year-old G2 P1001 female at 13 weeks’ gestation presented to the Emergency Department of Cho Ray Hospital, Ho Chi Minh City, Vietnam because of progressively worsening shortness of breath for the previous 5 days. She was in her usual state of health until 5 days prior to presentation, when she noticed uncomfortable breathing on exertion, fatigue, episodic chest pain, and palpitation. The patient had no significant past medical history and had taken no medications. She is a mother to a 3-year-old girl. Her previous pregnancy and vaginal delivery had no complications.
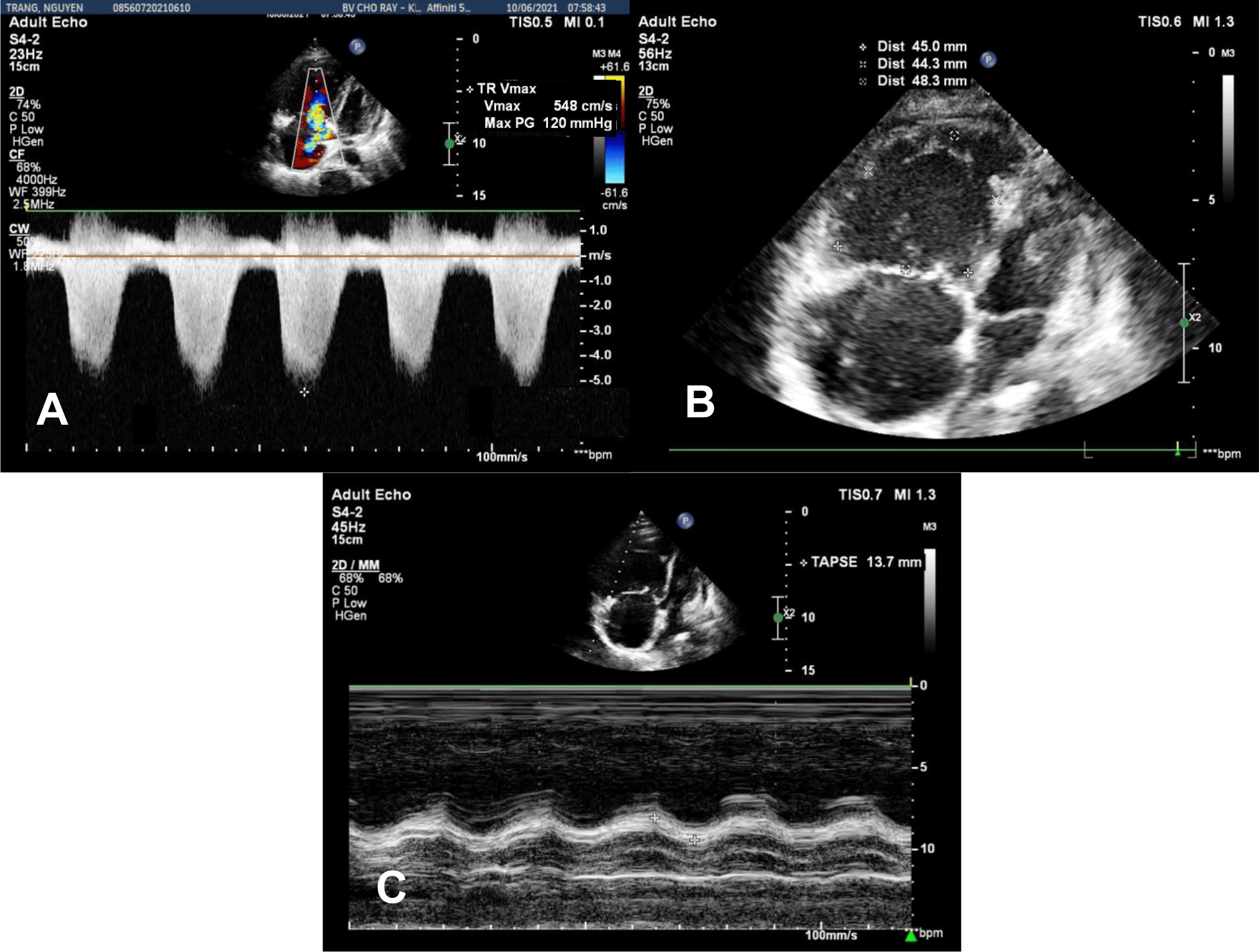
In the Emergency Department, physical examination revealed a blood pressure of 75/50 mmHg, heart rate of 110 bpm, respiratory rate of 32 bpm, and SpO2 of 85% on room air. The temperature was 37°C. Mild edema of the lower extremities was noticed. An initial diagnosis of acute respiratory failure and cardiogenic shock was made. Transthoracic echocardiography showed a left ventricular ejection fraction (LVEF) of 56%, mild pericardial effusion without cardiac tamponade, severe right ventricular (RV) dilation, tricuspid annular plane systolic excursion (TAPSE) of 13.7 mm, RV fractional area change (FAC) of 10.1%, and RV S Vel of 6.3 cm/s, severe tricuspid regurgitation with maximal tricuspid regurgitation velocity (TR Vmax) of 548 cm/s, severe pulmonary artery systolic pressure of 135 mmHg (Figure 1A, 1B, 1C). In addition, transabdominal ultrasound revealed a fetus consistent with 13-week gestation. The patient was admitted to our hospital during the COVID-19 pandemic. Her real-time PCR SARS-CoV-2 test was negative.
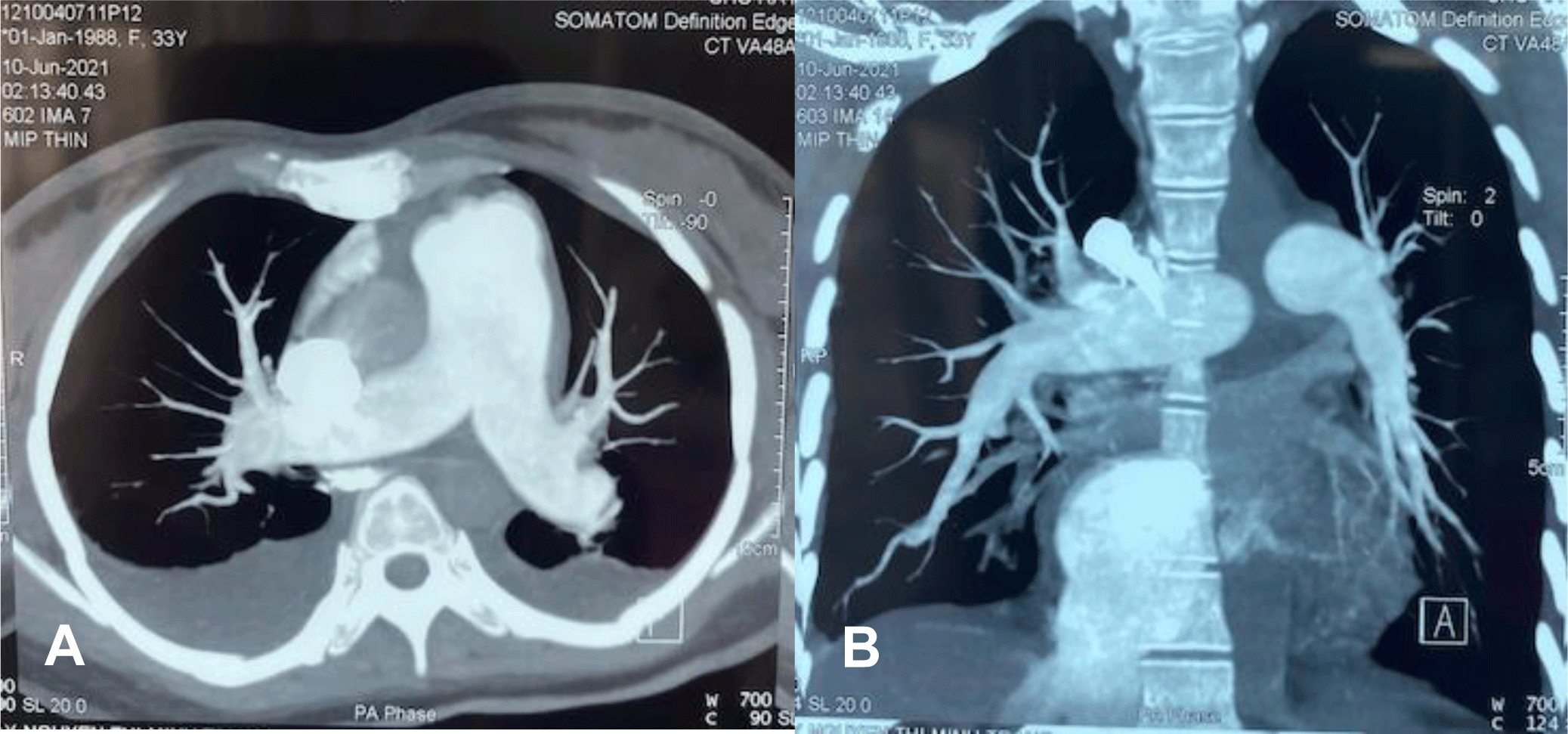
A vasoconstrictor agent, noradrenaline, and oxygen supplementation were immediately administered. Dobutamine was added to enhance the right ventricular contraction. At the same time, contrast chest computed tomography (CT) showed relatively mild pleural effusion and pericardial effusion without evidence of pulmonary embolism, enlarged right ventricle with the main pulmonary arterial diameter of 3.6 mm (Figure 2A, 2B).
Immunology work-up was performed according to the Vietnam National guidelines for pregnancy with pulmonary artery hypertension. Autoimmune markers confirmed SLE with hypocomplementemia, an elevation in Anti-dsDNA, positive Anti-nuclear antibody (ANA) (Table 1). Other autoimmune markers, including Anti-Sm/RO, anti-CCP were negative. Thrombocytopenia and autoimmune hemolytic anemia were documented. Her platelet count was 10 G/L, hemoglobin was 112 G/L with a positive directed Coomb test and a negative indirect Coomb test. Schistocytes on peripheral blood smear were below 1%. Urinalysis and kidney function were normal. Complete laboratory tests are shown in Table 1.
There were no clinical manifestations of SLE on physical examination. SLE was confirmed by ACR criteria. Cardiogenic shock as a result of acute right ventricular failure secondary to SLE-associated pulmonary hypertension was confirmed. Due to the severe nature of her condition, obstetrics, hematology, and rheumatology were consulted. After risks and benefits were discussed with the patient and family, termination of pregnancy was implemented.
The patient was transfused 1 kit of platelet targeting platelet count above 50 G/L to reduce the risk of major bleeding during dilation and curettage (D&C). ECMO was prepared in anticipation of cardiac collapse from an abrupt elevation in PAPs after removing the fetus; however, it was not utilized during the procedure. Changes in hemodynamic parameters before and during the use of positive ionotropic agents, and before and after termination of pregnancy were shown in the Table 2.
The patient’s condition stabilized after the procedure. Oxygen saturation was 95% in room air. Noradrenaline and dobutamine were weaned off. Corticosteroids therapy with solumedrol 80 mg was given per rheumatology and hematology recommendations. After inotropic agents were stopped, bosentan was added to lower pulmonary artery pressure. Furosemide and pantoprazole were also given.
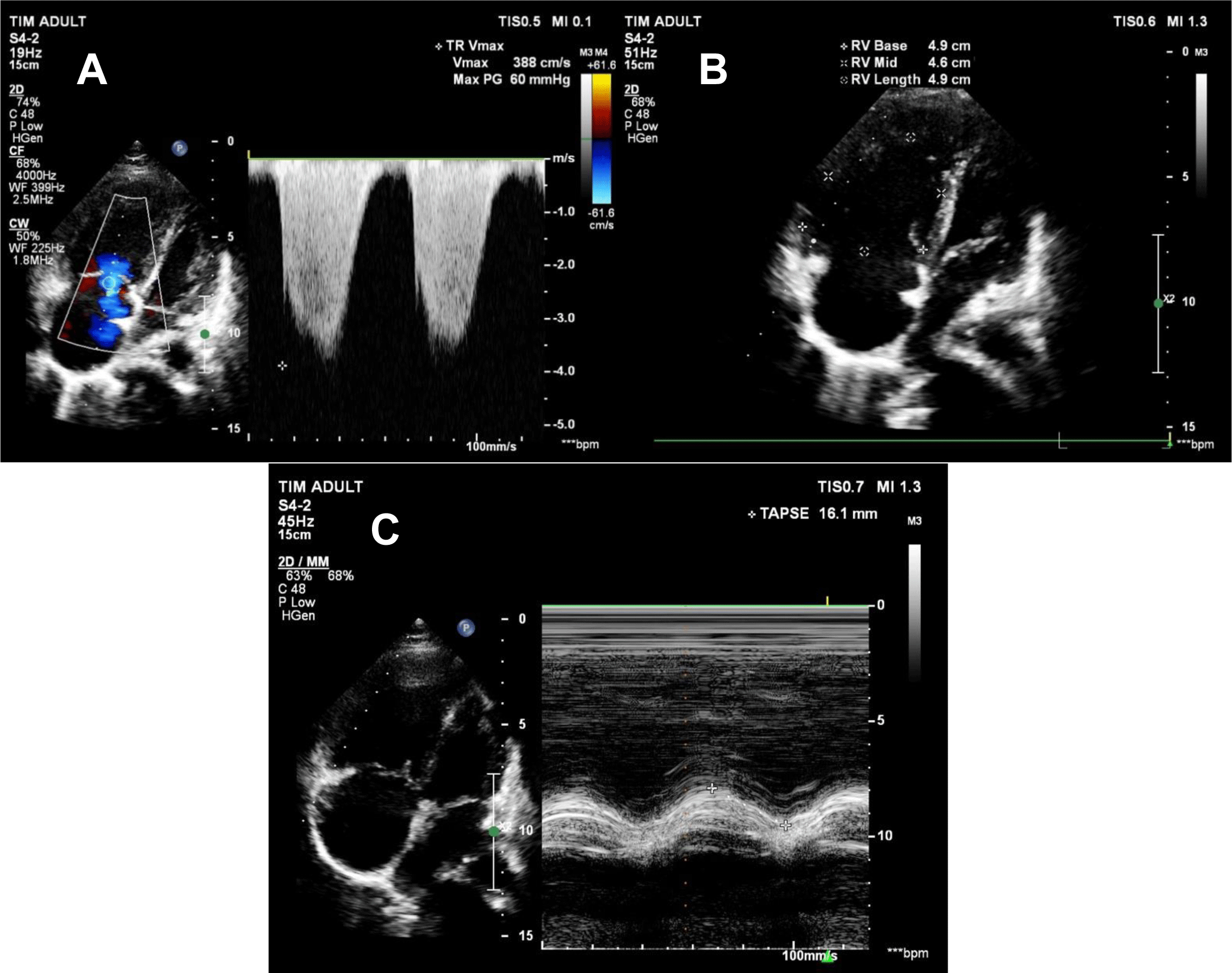
The patient remained stable, and there was a downward trend in pulmonary artery pressure on transthoracic echocardiography. On day 5 and day 10 of her hospital course, PAPs were 84 mmHg and 65 mmHg, respectively. TR Vmax was 388 cm/s, TAPSE was 16.1 mm, FAC was 26%, and RV S Vel was 9.9 m/s (Figure 3A, 3B, 3C). As PAPs decreased, right ventricular function significantly improved. The patient could sit up and supportive oxygenation was weaned off. She was discharged on day 11 after admission. Medications on admission included methylprednisolone 16 mg 3 tablets QD, bosentan 125 mg BID, hydrochloroquine 200 mg BID, verospirone/furosemide 50/20 QD.
3. DISCUSSION
This was a complicated case that required multidisciplinary care from cardiology, rheumatology, hematology, and obstetrics. In our opinion, there are four major issues related to the clinical practice in cases like this, including high PAPs presented as an initial manifestation of SLE in pregnancy, choosing the suitable time for termination of pregnancy, and management of pregnant patients with PAPs-associated SLE.
First, many retrospective studies have reported a discrepancy in the prevalence of PAH-associated SLE. The prevalence has been higher in Asian countries than in Western countries. According to previous analyses, PAH-associated SLE was 5% and 23% in South Korea and China, respectively [3-4]. To our knowledge, SLE is a complex disease with various organ related. Common pulmonary manifestations in SLE are pleuritis, interstitial tissue disease, vascular disease. SLE-associated PAH has many documented mechanisms, including thromboembolic consequences, progressive autoimmune vascular systems, and disorders in vasoconstriction, and vasodilation of the pulmonary vasculature [5]. In addition, dynamic physiological changes in healthy pregnant women also contribute to right ventricle dysfunction secondary to pregnancy-induced PAH. A significant hemostatic change occurs during the second trimester of pregnancy [6]. In the present case, the symptoms of fatigue, shortness of breath, and palpitation occurred at 13 weeks of gestation, consistent with documented physiological changes in pregnancy. More likely, SLE-associated PAH and hemostatic changes in pregnancy had a cumulative effect in elevation of PAPs in this patient.
We want to emphasize the extremely high pulmonary artery systolic pressure in this case. To the best of our knowledge, this patient’s is the highest systolic PAP (135 mmHg) that has been reported in the literature. There were no clinical manifestations of SLE on physical examination, such as discoid lupus and malar rash found. A contrast chest CT was performed to rule out thromboembolic events as a cause of elevated PAPs. Therefore, PAH was considered the initial manifestation of SLE in pregnancy. Commonly, PAH in SLE manifests in the typical presentation of SLE. Severe pulmonary hypertension as an initial manifestation of SLE has been seldom reported in the literature. Prete et al. reported a 32-year-old Moroccan woman with severe PAH as the initial manifestation of SLE, with systolic PAP of 90 mmHg on transthoracic echocardiography [7]. Kiani et al. also reported a young female who presented with severe PAH with right ventricular failure leading to cardiogenic shock and was found to have SLE, with sPAP 81 mmHg on admission. In both cases, the patients were not pregnant [8]. In our case, PAH was the initial manifestation of SLE in a 13-week pregnant woman with SLE and severe PAH leading to acute right ventricle failure and cardiogenic shock. Since severe PAH is a rare initial presentation of SLE, severe SLE-associated PAH in pregnant patients presenting as the initial manifestation of SLE is extremely rare.
Second, our patient was transferred to the Emergency Department with an unstable hemostatic condition caused by acute right ventricular failure. A study conducted in the US showed a 56% mortality rate in pregnant patients with secondary pulmonary artery hypertension [9]. Both SLE and pulmonary artery pulmonary have traumatic effects on indirect maternal deaths in the United Kingdom [10]. After consulting specialists in Rheumatology, Hematology, and Critical Care Medicine, risks and benefits were considered carefully and discussed with the patient and family. Termination of the gestation was chosen to reduce strain for the right ventricle to save maternal life. After the fetus was removed, a large amount of blood volume would return rapidly from peripheral veins to the right ventricle. These abrupt hemodynamic changes may lead to a rapid increase in PAPs, and the patient would face the risk of cardiovascular collapse. In clinical practice, the risks of continuing or termination of pregnancy should be considered carefully regarding maternal death probability and patient’s expectation. Team ECMO was on standby to support in the worst-case scenario [11].
Third, we would like to highlight options for the treatment of SLE-associated PAH in pregnant women. First, if women in child bearing age have pulmonary artery hypertension secondary to SLE, they must be explained potential risks that they would face if they become pregnant. Then they have to consistently use contraceptives to prevent pregnancy. If a patient with SLE-associated PAH still wishes to be pregnant, she must understand and accept potential risks of fetal and maternal death during pregnancy. For example, a flare-up of SLE can occur more frequently with different injuries to targeted organs such as kidney, hematology, and neurology. Furthermore, some medications such as bosentan, which is given in treatment of PAH, are pregnancy category X, which is an absolute contraindication. As mentioned above, these pregnancies need to be managed coordinately with rheumatology, cardiology, and obstetrics specialists. In our case, the patient had a non-significant past medical history. SLE-associated PAH was considered as her initial presentation. Different therapeutic strategies were proposed based on the patient’s hemostatic status and her expectation. Our patient presented at emergency department with acute respiratory failure and advanced shock due to SLE-associated PAH. As described above, termination of pregnancy was considered an optimal option at the moment. Kawabe et al. reported a pregnancy with SLE-induced PAH that was managed successfully and was delivered at 28 weeks of gestation by Cesarean section [12]. Their patient was diagnosed with typical SLE and SLE-associated PAH before pregnancy. Indeed, the patient was successfully treated and recovered. She was admitted to the authors’ hospital when she was at 26-week gestation. According to these authors, her vital signs were relatively stable, there was no sign of right ventricular failure, and the highest estimated sPAP was recorded at 68 mmHg. The authors decided to perform Cesarean section at 28 weeks. Both mother and infant were in good health.
Controlling the activity of SLE and termination of the pregnancy had a pivotal role in the management of PAH in the presented case [13]. In our patient, follow-up TTE showed a gradual reduction in the severity of PAPs. Furthermore, vasodilators also significantly contributed to the decrease of PAPs. Endothelin-1 is a potent vasoconstrictor, which also mediates cell proliferation, fibrosis and inflammation. Bosentan is a competitive inhibitor of endothelin-1 receptors. Oral endothelin-1 receptor antagonists are used for patients with PAH Group I, which includes SLE-associated PAH according to WHO classification [14]. In our patient, bosentan was given when dobutamine had been discontinued for 24 hours.
Conclusion
Severe PAPs may present as an initial manifestation of SLE in pregnant women who have been diagnosed with SLE. Gestational termination is considered a referred approach for pregnancy with SLE-associated PAH who have signs of acute right ventricular failure. Women in childbearing age with SLE- associated PAH should be counseled to avoid pregnancy. For women who still wish to become pregnant, they and their families must be counseled and accept the potential risks of pregnancy. In these cases, multidisciplinary management for pregnancy with SLE-associated PAH is considered an appropriate approach to improve maternal and fetal outcomes.