1. INTRODUCTION
Aortic valve stenosis is one of the most common valvular heart diseases in patients with end-stage chronic kidney disease, including chronic dialysis [1]. Although surgical aortic valve replacement (SAVR) is considered the standard therapy for aortic stenosis (AS), the mortality rate is quite high for patients with AS and chronic dialysis who undergoing open surgery because of its comorbidities [2]. In recent decades, TAVR has emerged as a potential alternative to SAVR for the treatment of such patients [3, 4]. The majority of TAVR was performed via the femoral artery in patients with the mean age of 75 years or older; other approaches apart from the femoral artery and in patients in the age group of 60 years are rarely mentioned. In the literature, there have been no reports of TAVR through the carotid artery in patients at 60 years of age with severe AS and chronic dialysis. In this report, we describe a case of severe AS and chronic dialysis who underwent transcarotid TAVR.
2. CASE REPORT
A 60-year-old man was admitted to our hospital due to chest pain and dyspnea with mild exertion (NYHA class III). He had a history of severe AS within 5 years, an end-stage renal disease requiring chronic dialysis, hypertension, chronic heart failure, diabetes, asthma, and severe frailty. Transthoracic echocardiography showed a severe AS with 87 mmHg peak gradient, 55 mmHg mean gradient, and 0.8 cm2 surface area. The left ventricular ejection fraction was 28%. Electrocardiography (ECG) showed a left bundle branch block. Coronary angiography revealed no significant stenosis. He was considered at high risk for open surgery because of his comorbidities. The logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) II and Society of Thoracic Surgeons (STS) scores were 9.42% and 6.9%, respectively. Therefore, TAVR was considered for such operative risk and his refusal of surgery. The final decision to use TAVR was made after a careful discussion between the heart team and the patient and his family.
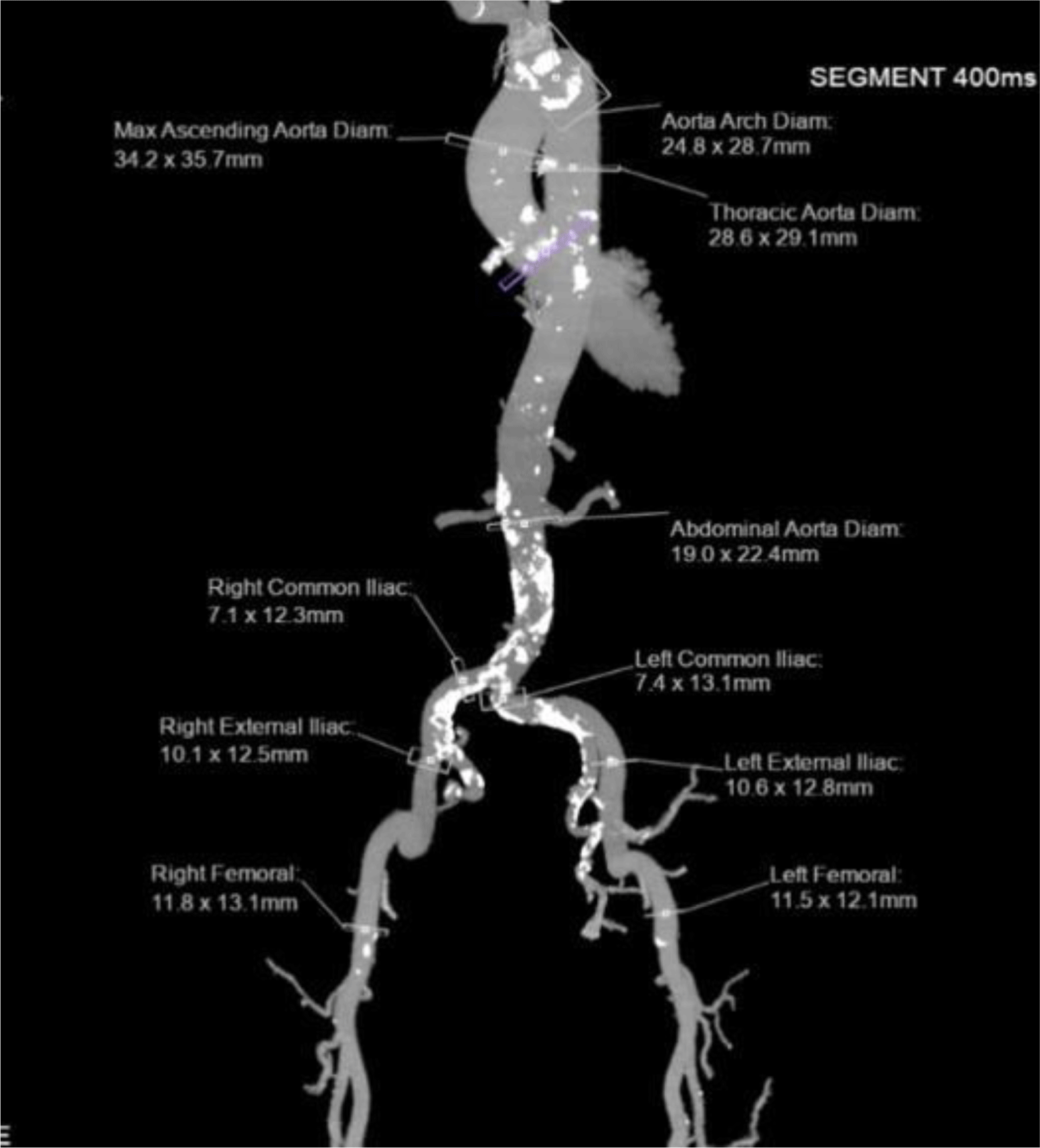
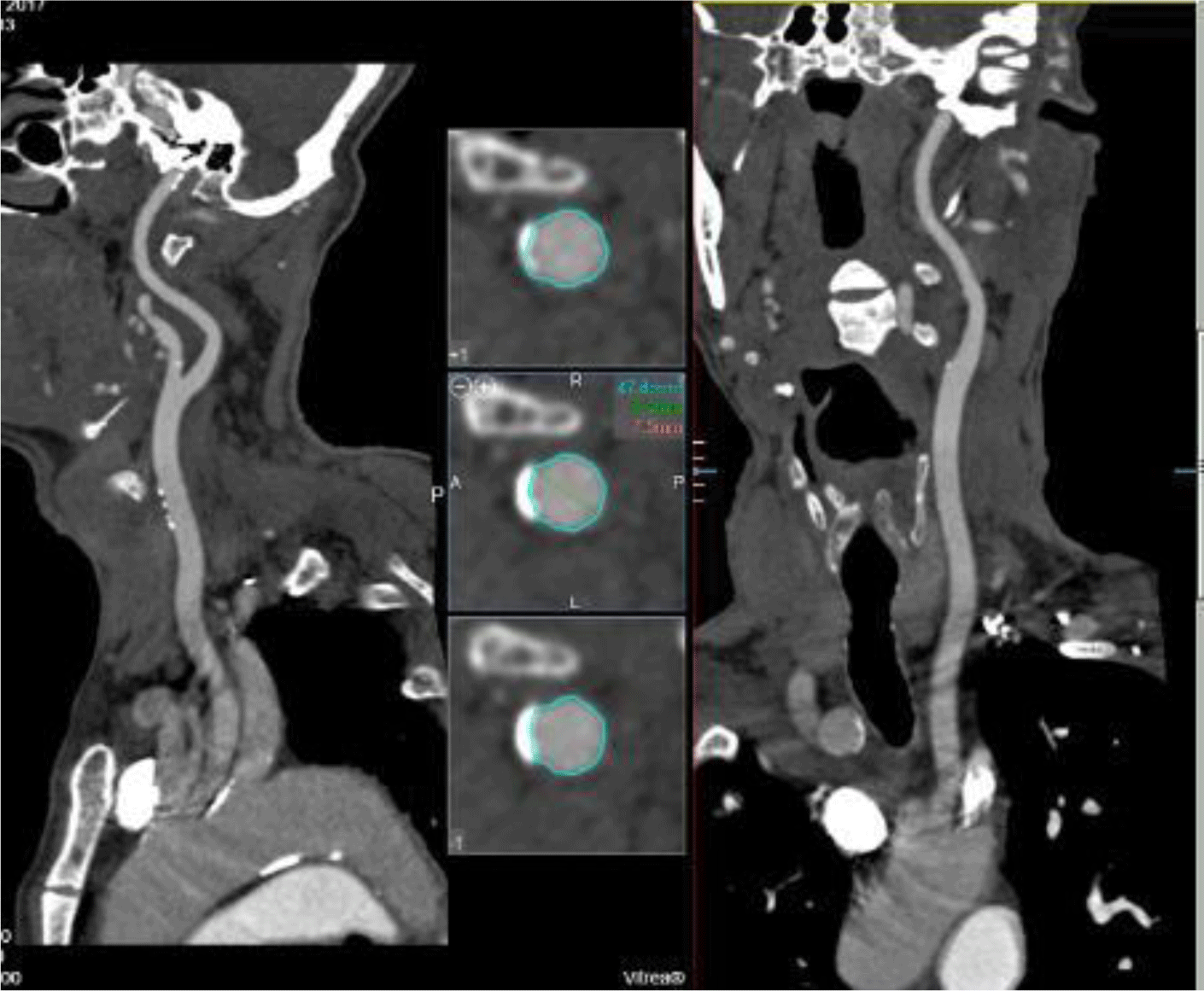
Computed tomography (CT) angiography showed that bilateral iliofemoral arteries were tortuous, heavily calcified, and stenosed. The diameters of the left and right common iliacarteries at their narrowest sections were 7.1 mm and 7.4 mm, respectively (Figure 1). Due to the unfavorable femoral approach, we evaluated the subclavian and carotid artery accesses. The diameters of bilateral subclavian arteries were appropximately 5.4mm. The right and left common carotid arteries had mild calcification with diameters of 8 and 8.2 mm, respectively (Figure 2). The cerebral arteries were normal. The heart team chose the left carotid approach to implant a CoreValve Evolut R System (Medtronic Inc, Minneapolis, Minnesota, USA) since the transfemoral access was not appropriate.
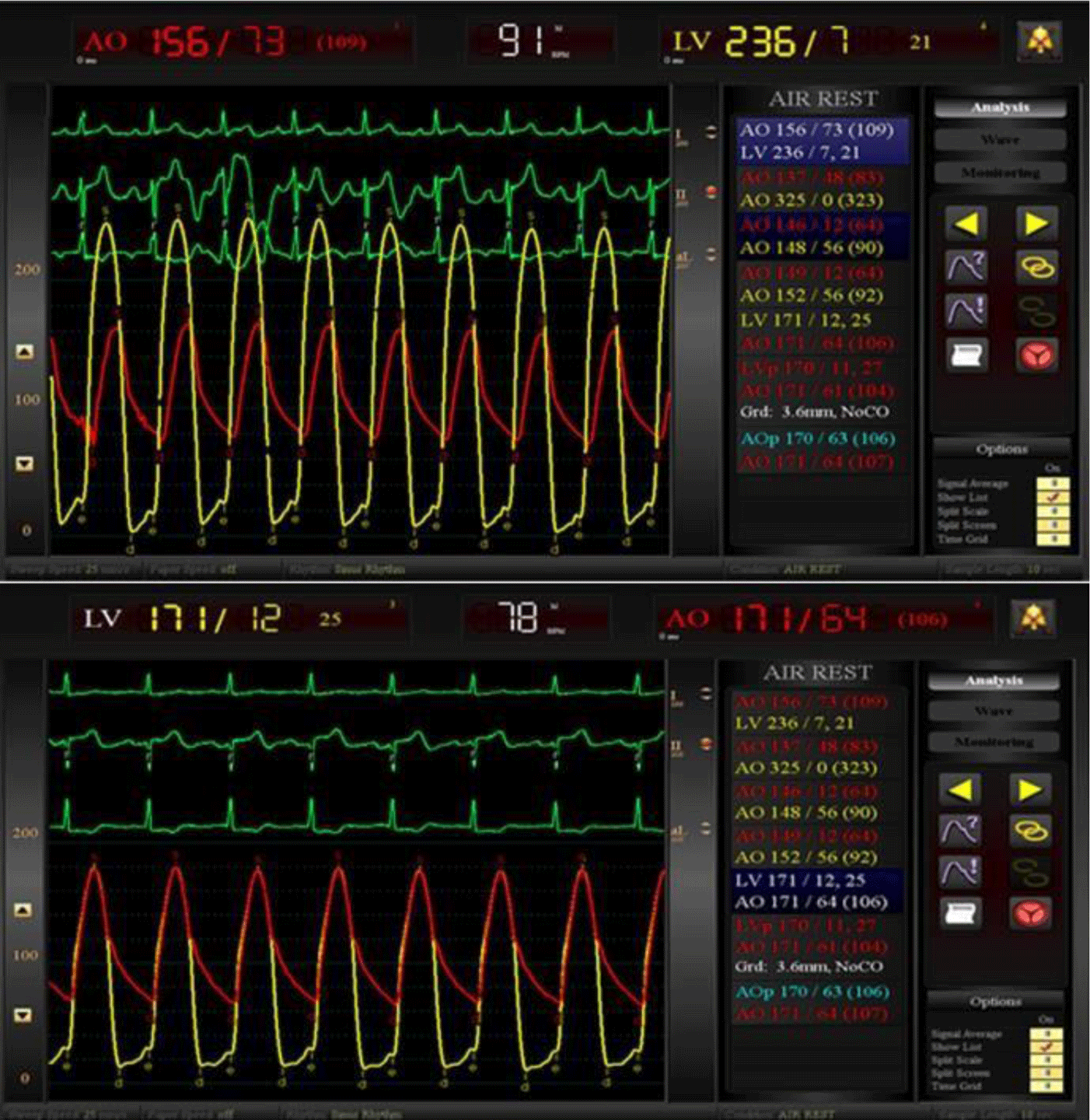
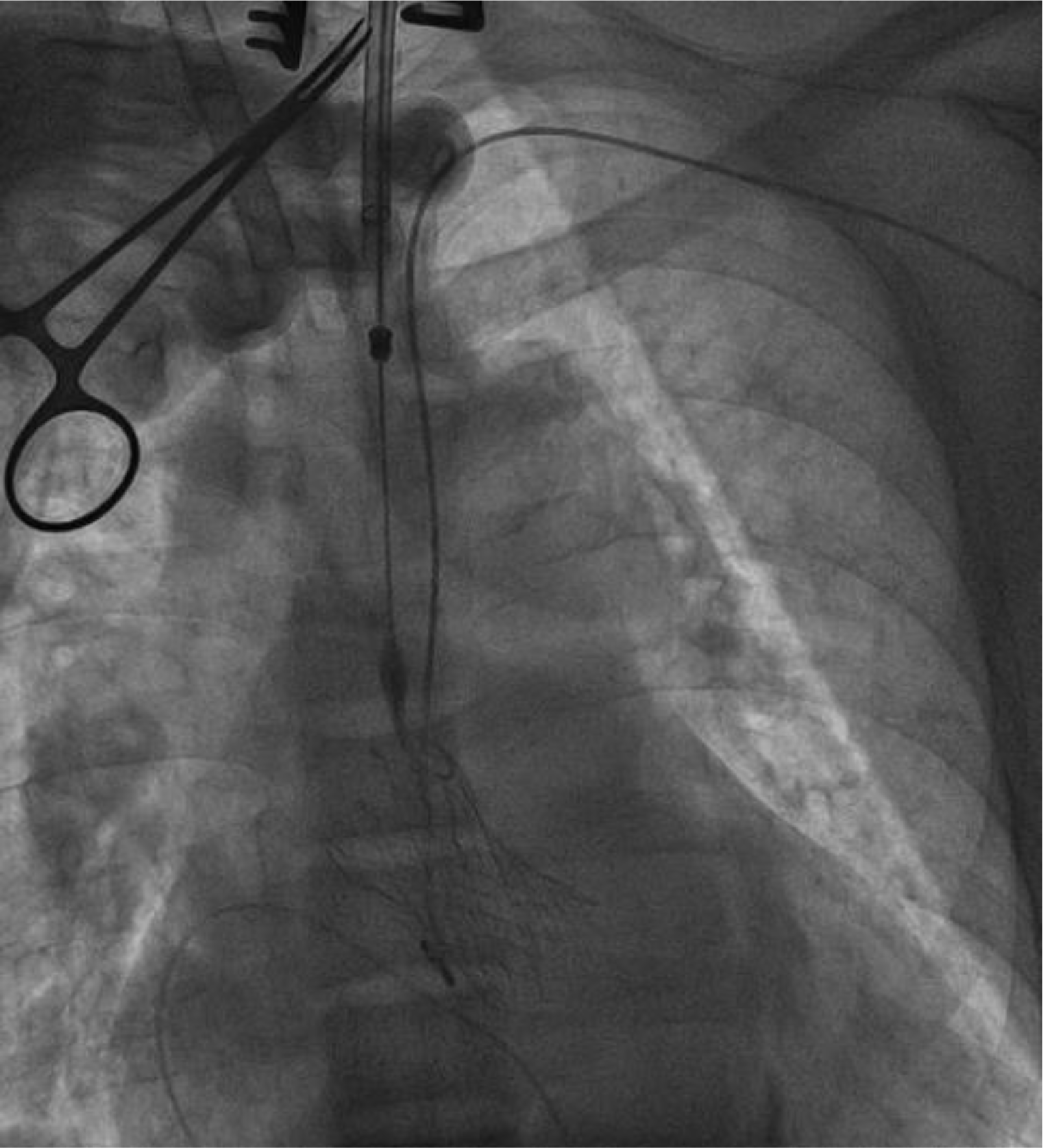
The patient received dialysis before the procedure. He underwent TAVR under general anesthesia with transesophageal echocardiography (TEE) guidance in a hybrid room. A temporary pacemaker was implanted via the right femoral vein. First, we punctured the left radial artery to introduce the first 6F pigtail catheter, positioned it in the non-coronary aortic sinus, and connected it to a blood pressure monitor. The left common carotid artery was surgically exposed to insert a 7F introducer sheath into the artery, then replaced by a 14F introducer sheath (Sentrant, Medtronic). With the support of an Amplatz catheter, a straight-tip guidewire (Terumo) crossed the aortic valve into the left ventricle and was then replaced by the second 6F pigtail catheter. Before prosthesis valve replacement, we evaluated the hemodynamic performance of the aortic valve (Figure 3). The pigtail catheter in the left ventricle was replaced by a 0.035” x 260 cm guidewire (Confida, Medtronic). Then, the 14F sheath was removed to insert the 29-mm CoreValve Evolut R system. During rapid ventricular pacing at 180 bpm, we delivered the Evolut R system under fluoroscopy and successfully implanted it at the correct position of the aortic annulus (Figure 4). TEE and aortic root angiography confirmed the accurate position of the prosthetic valve without any regurgitation or transvalvular gradient. All the instruments were removed, and the arterial access sites were repaired surgically. The patient was extubated 2 hours after the procedure. The temporary pacemaker was removed after 24 hours. There were no postoperative arrhythmias or surgical site complications. He was discharged after 7 days, and the carotid artery was evaluated by ultrasonography before discharge. After 12 months of follow-up, his symptoms significantly improved from NYHA class III to class I without any major cardiovascular adverse events recorded.
3. DISCUSSION
According to the randomized controlled trials published in recent years, TAVR is non-inferior or even superior to SAVR in patients with severe AS, regardless of surgical risk [5]. However, patients with severe renal impairment (including dialysis) were not included in these studies. Therefore, the decision to TAVR in such patients is a challenge, even though this group of patients often presents with a high surgical risk because of many comorbidities. To date, some retrospective studies and pooled analyses have shown that dialysis patients who received TAVR had a lower rate of 30-day mortality and a similar rate of 1-year mortality when compared to SAVR. However, TAVR patients required more permanent pacemaker placement while SAVR patients had longer hospital stays [3, 4]. TAVR in dialysis patients showed a significantly higher rate of complications (such as major bleeding, and permanent pacemaker placement), short-term and long-term mortality than in non-dialysis patients [5]. Our patient is a 60-years-old man with symptomatic severe AS, chronic dialysis, and many comorbidities. Despite the high surgical risk and the patient’s rejection of the surgery, the heart team has discussed these issues thoroughly with one another and, importantly, with the patient, because the evidence for TAVR in dialysis patients is still limited. In addition, one of the issues that were discussed carefully is our patient’s age, which is considered “young” compared to the general population of TAVR in the world aged ≥ 75 years. Evidence for the efficacy of TAVR in patients below 75 years old is uncertain. The YOUNG TAVR Registry has shown that, compared with the patients aged ≥ 76 years, the patients aged ≤ 75 years undergoing TAVR had a significantly higher rate of the bicuspid aortic valve, chronic lung disease, diabetes, coronary heart disease, and low ejection fractions. There was no significant difference in mortality within 30 days and 1 year between the two age groups, however, mortality at 2 years was significantly higher in patients aged ≤ 75 years [6]. The 2020 AHA/ACC guideline for heart valve disease recommends that if the patients are below 65 years old with AS who are at low or moderate surgical risk, SAVR is selected; however, if such patients with life expectancy > 1 year and have high surgical risk or inability to have surgery, TAVR is strongly recommended, regardless of age [7].
The transfemoral access is considered the standard approach of TAVR. However, there are about 30% of patients in whom the femoral artery route is inappropriate for this procedure because of the unsuitable iliofemoral artery size, tortuous path, and heavily calcified vessels [8]. In recent years, transcarotid (TC) access is gradually becoming a promising alternative approach when the femoral artery is unsuitable [9]. The first TAVR using transcarotid artery was reported in 2010 by Modine, after which the number of cases has been increasing [10]. This technique allows a direct path for aortic valve implantation as well as provides a shorter distance from the entry access to the aortic root, which gives a better device control for valve implantation. To perform TAVR via TC, the diameter of the common carotid artery should be ≥ 7mm, and the left carotid access is preferred to the right one because it found superior coaxial alignment of the transcatheter aortic valve with the aortic annulus [8]. Stroke is the most noticeable complication when performing TAVR through the carotid artery. The main cause is embolism due to atherosclerotic debris from the carotid artery or valvular calcification. There are some methods to help reduce stroke in TC, such as a thorough assessment of the bilateral carotid arteries, the circle of Willis by CT or magnetic resonance angiography, closely monitoring the cerebral oxygen saturation during the procedure, clamping the distal carotid artery during valve deployment [11]. Our patient had heavily calcified, and tortuous iliofemoral arteries, which were considered inappropriate for the transfemoral route, in addition, the diameter of subclavian arteries was not suitable for TAVR, thus, we chose the carotid artery as an appropriate approach to perform this procedure.








