1. INTRODUCTION
The onset of secondary sexual characteristics in girls before the age of 8 and boys before the age of 9 has been defined as precocious puberty [1]. Central precocious puberty (CPP) is involved in the early activation of pulsatile gonadotropin-releasing hormone secretion, while the early pubertal maturation, which is independent of the activation of the hypothalamic-pituitary-gonadal axis, constitutes peripheral precocious puberty [1]. Impaired adult stature in CPP patients caused by early fusion of epiphyseal growth plates is the primary concern among doctors and families [2]. Thus, GnRHa therapy, which downregulates the hypothalamic-pituitary-gonadal axis, has been widely indicated for the treatment of CPP. For more than 20 years, several studies aimed to determine the effect of GnRH therapy on final height; however, there is no evidence from randomized controlled trials. According to data from 400 CPP girls, Carel et al. demonstrated that the mean height advancement ranged from 3 to 10 cm [3]. The efficacy in height advancement is varied among studies, with the undisputed height gain benefit in progressive CPP before the age of 6 [4]. These days, controversy remains regarding the optimal use of this therapy in CPP patients, especially the CPP girls who visit the hospital after age 8. Most previous research on CPP in Vietnam have focused on the characteristics of epidemiology, causes, clinical signs, and a few studies evaluated the short-term effect of the treatment [5-7]. Therefore, we conducted this study to evaluate the effect of this therapy on the pubertal progression, auxological outcomes (BMI changes and predicted height gain benefit) and bone density of CPP patients in both genders at the end of the treatment.
2. MATERIALS AND METHOD
This research was conducted at the Department of Nephrology and Endocrinology, Children Hospital 2, Ho Chi Minh City, Vietnam. We recruited children who finished treatment from the 1st of June 2011 to the 1st of June 2021. The duration of data collection ranged from the 1st of December 2020 to the 1st of June 2021.
This was a cross-sectional design. The reporting of this study was checked against the STROBE checklist for cross-sectional studies [8].
At the outpatient clinic archive of the Children Hospital 2, we collected data from the medical records of CPP patients who completed GnRHa from the 1st of June 2011 to the 1st of June 2021. Inclusion criteria included the patients who reached the indication of GnRHa therapy following Children’s hospital 2 protocol: progressive CPP was diagnosed under 6 years, progressive CPP in other age which impaired predicted adult height, prevent psychological and/or behavioral problems related to CPP; finished the treatment with Triptorelin from the 1st of June 2011 to the 1st of June 2021 (Patients were administered triptorelin 3.75 mg every 4 weeks, subcutaneous injection. Treatment was stopped when the calendar age reached 10 to 11 years or bone age reached 12 to 13 years). Exclusion criteria consisted of patients who have been followed-up irregularly, with peripheral precocious puberty, hypothyroidism, and medical records which lack essential information.
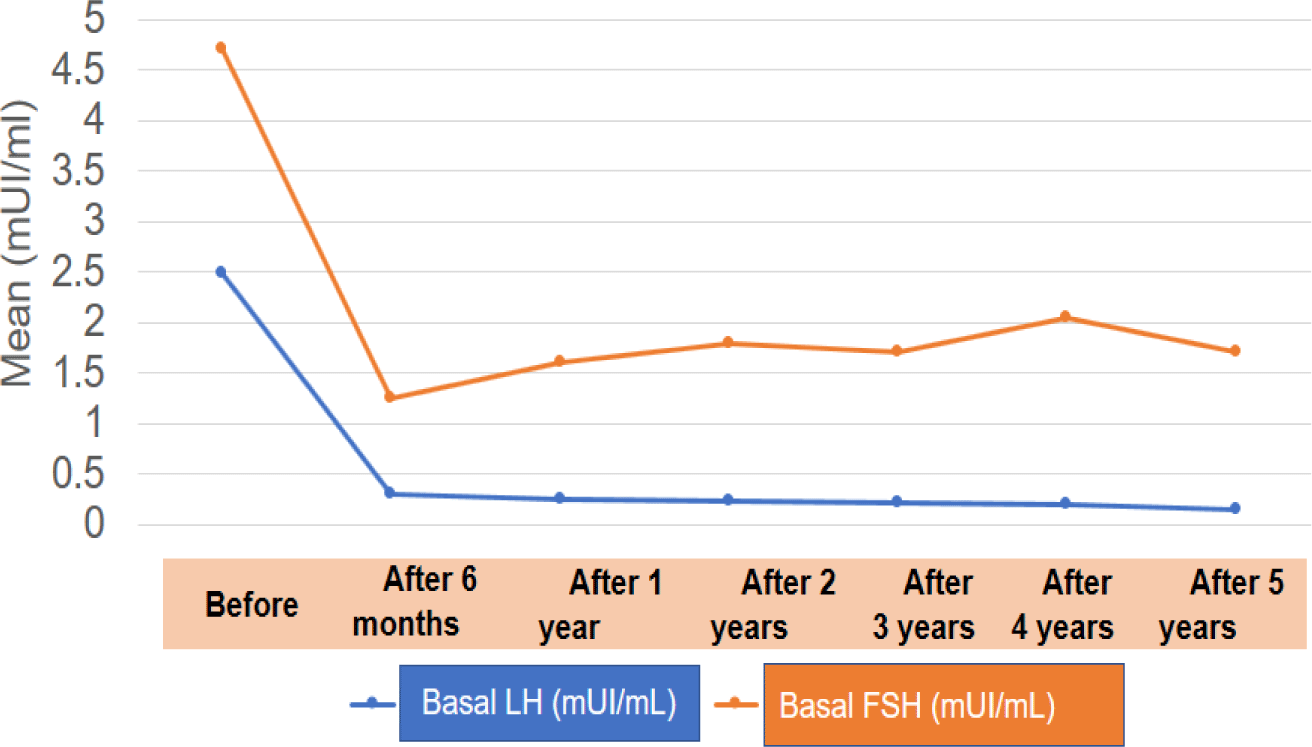
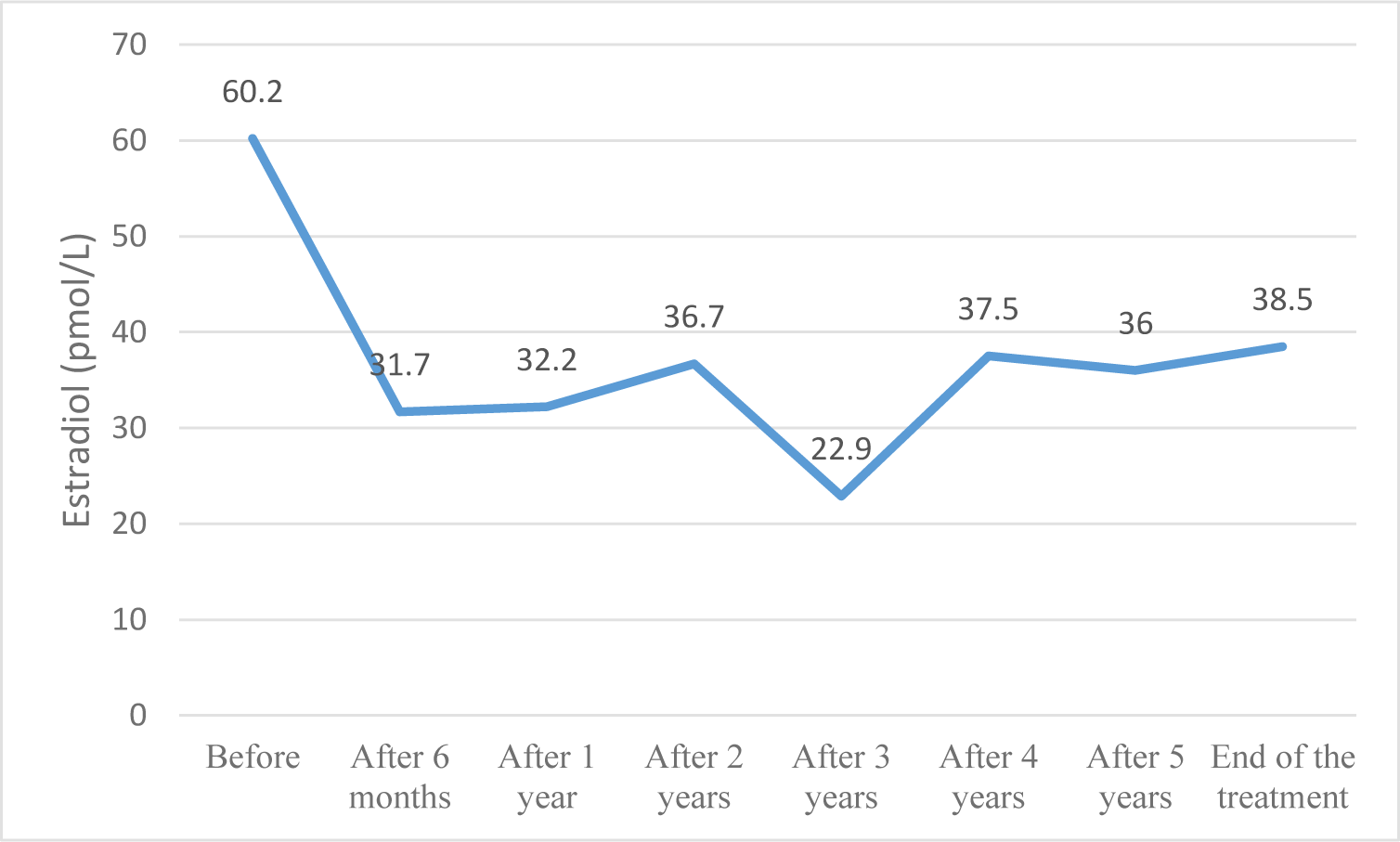
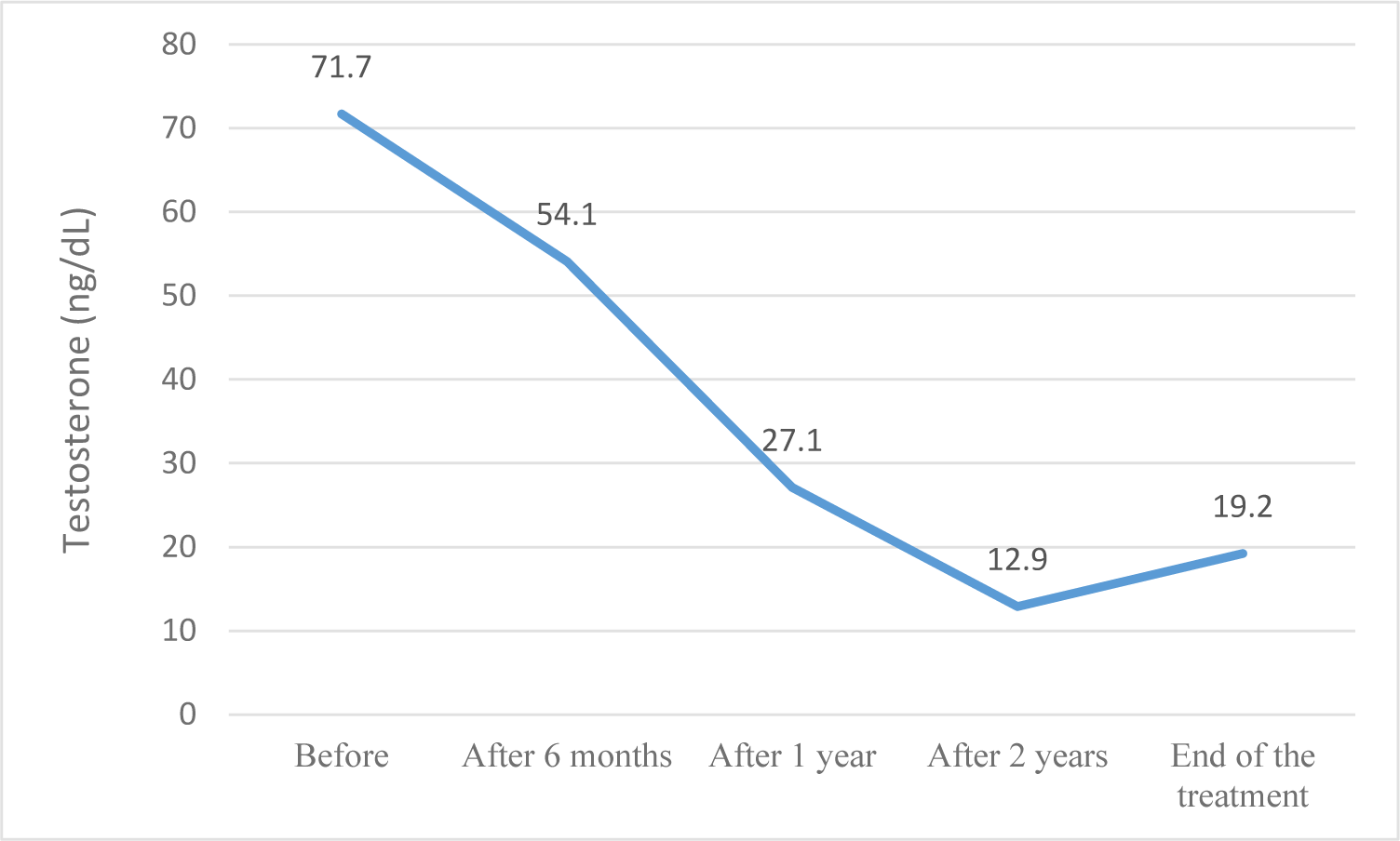
At the outpatient clinic of this center, we chose the patient who finished GnRHa therapy in the study period. Height, weight, bone age, sex characteristics, Luteinizing hormone (LH) concentration, follicle-stimulating hormone (FSH) concentration, serum estradiol, and testosterone were assessed after 6 months and each year of the treatment. The breast, genital development and pubic hair were evaluated by pediatric endocrinologists according to the Tanner staging of puberty [9]. A radiograph of the left hand was performed every 6 months to determine BA using the Greulich-Pyle method [10]. The Bayley-Pinneau method was used to estimate the predicted adult height (PAH) [11]. Final height prediction was calculated by dividing the current height by the decimal fraction based on bone age [12]. We compared their eventual PAH with their initial PAH. The body mass index (BMI) was calculated based on height and weight (kg/m2). Also, we investigated bone mineral density (BMD) and bone metabolism in these patients at the end of the treatment.
The sample size of this study was calculated based on the following formula:
In that formula: tolerated margin of error d = 0.04, type I error is = 0.05 for a confidence level of 95%, so Z(1-α/2) = 1,96 is 1.96 and the proportion of CPP patients who successfully respond to GnRHa at the treatment discontinuation from data of the previous study was p= 94% [13].
The minimum sample size of the study is N = 136.
Data were input by Epidata version 13 and analyzed using Stata version 14 software, presenting as means and standard deviations or medians and quartiles. We compared the results between groups using the paired t-test or Wilcoxon signed rank-sum test. We considered P < 0.05 to be statistically significant.
This study was approved by the local ethics committee of Ho Chi Minh Medicine and Pharmacy University with registration number 544/HÐÐÐ-ÐHYD and Children Hospital 2 with registration number 53/20-BVND2. This study was eligible for Expedited Review. Informed consent was not collected from the patients who had finished the treatment before the beginning of the study. We obtained informed consent from the parents in the group of participants who did not complete GnRHa therapy at the start of the research and discontinued this treatment before the 1st of June 2021.
3. RESULTS
From the 1st of June 2011 to the 1st of June 2021, data of 140 girls and 3 boys were collected. When we started to collect data, 132 patients had already finished the treatment. 11 patients had started the GnRHa treatment before the time we conducted this study, and all of them discontinued the therapy before the 1st of June 2021.
The most common symptoms were breast development in females, and pubic hair, and axillary hair in males. 17.9 % of girl patients had already started menstruation at the initial time of the treatment. In males patients, mean testicular volume: 8.2 ± 1 ml (median: 8.5, range: 7-9 ml); mean penis length: 6.7 ± 0.6 cm (median: 6.5, range: 5.5-7.2 cm).
10% of cases had an abnormality in brain MRI, and microadenoma was the most common lesion.
The mean duration of the regimen was 2.4 ± 0.6 years (median: 2.4; IQR: 1-2.8 years; range: 1-5 years).
The mean age at the eventual treatment time was 10.4 ± 0.5 years (median: 10.4 years; IQR: 10-10.5 years; range: 9.8-11.2 years), and the mean BA was 11.4 ± 0.1 years (median: 11 years, IQR: 11-12 years, range: 11-14).
The pre-treatment PAH was 157.8 ± 0.6 cm in girls and the PAH at treatment discontinuation (162.0 ± 0.5 cm in girls) was significantly higher than the initial PAH (p<0.05). There was an increment in the PAH after GnRHa therapy in male patients, with an average height gain of 2.9 ± 3.3 cm (p =0.4). To analyze whether auxological outcomes were affected by age at the beginning of treatment, we separated girl patients into three age groups, patients treated under 6 years of age (n = 4), patients who started this therapy from 6 to 8 years of age (n = 56) and children who were indicated for GnRHa after 8 years of age (n = 83). For girls with GnRHa therapy before 6 years of age, the treatment resulted in a predicted average gain in adult height of 10.2 ± 3.2 cm (p = 0.049), whereas for those treated between 6 and 8 years of age, treatment resulted in a predicted height advancement in adult height of 5.3 ± 0.7 cm (p<0.05). The predicted average height gain of girls aged over 8 years was also remarkable, 3.2 ± 0.6 cm (p<0.05).
In the group of girls who had menstruation before, cessation of menstruation happened in all of them after treatment. The proportion of females with breast development suppression was 97 %, and this result was maintained until the end of the treatment. There were no significant changes in pubic hair and axillary hair.
In boys, testicular volume decreased during the treatment period, from 8.2 ± 1 ml to 7.5 ± 0.9 ml at the last time of the treatment (p= 0.17). There were no significant changes in pubic hair and axillary hair.
After GnRHa indication, the mean BMI in the girl group significantly increased while BMI SDS did not change significantly and these changes were presented in Table 4. The height velocity decreased significantly to 4.2 ± 2.3 cm after treatment (p<0.05). The rate of bone age advancement also declined, with the difference between BA and CA decreasing from 2.4 ± 0.1 to 0.8 ± 0.3 years (p<0.05).
GnRHa therapy was generally well-tolerated in the short term, without any local adverse events. In the first 3 months of the regimen, small withdrawal bleeds occurred in 5% of girls.
At our center, the measurement of BMD and biochemical markers of bone metabolism are not indicated regularly in CPP patients. When we started collecting data, 132 patients had already finished the treatment; thus, we did not have data about these laboratory tests from these participants. 11 patients did not complete the therapy when we started to conduct this study. We indicated BMD and biochemical markers of bone metabolism in these participants at the treatment discontinuation. The parameters about bone mineral density (BMD) and biochemical markers of bone metabolism (serum Calcium, phosphorus, vitamin D, alkaline phosphatase, parathyroid hormone) had shown no abnormality (Table 5).
4. DISCUSSION
GnRHa therapy resulted in regression or halting of pubertal progression and a reduction in growth velocity after 6 months; this result was maintained until the end of the treatment. Due to the secretion of the adrenal glands, pubic hair and axillary hair showed no significant changes. When the pulsatile Gonadotropin-releasing hormone secretion was suppressed, estradiol and testosterone also returned to pre-pubertal levels. This result is similar to other studies [5, 13]. From a sample of 457 children with CPP, Le Ngoc Duy reported that the proportion of girls with breast development suppression was 97%, and this suppression was maintained during the treatment period [5]. Lee et al. also demonstrated that GnRHa successfully maintained LH suppression in all patients throughout the study period [13].
In terms of height gain benefit, we compared the initial height prediction with the height prediction at the end of the treatment based on the Bayley-Pinneau method. This method was first introduced in 1959 and has been become the most popular method for final height prediction in pediatric endocrinology. Several studies have evaluated the accuracy of this method. In 1995, Bar et al. reported that the predicted height calculated by the Bayley-Pinneau formula was correlated significantly with final adult height (r = 0.85;p <0.001) [11]. This study concluded that the method could be reliable in the prediction of adult height in CPP girls. On the other hand, Kirkland et al. reported that this method might be faulty, with the range of error was 6.6±1.5 cm [14]. According to data from Turkish children, Tarim observed that Bayley-Pinneau method is not accurate and frequently leads to an overestimation of the final height of CPP patients [15]. It is a fact that there is no best formula for forecasting final adult height in children. Despite the modest accuracy, until now, Bayley-Pinneau was the most reliable method and still used for important treatment decisions and research in the field of pediatric endocrinology, not only precocious puberty but also other conditions: short stature, treatment for transgirls, congenital hypothyroidism, ect. According to a consensus statement about CPP treatment by the European Society for Paediatric Endocrinology, the decision of whether to treat CPP children with GnRHa depends upon the child’s age, the rate of pubertal progression and the estimated adult height based on the Bayley and Pinneau method [4]. In the research field of CPP, there is no evidence from randomized controlled trials that demonstrated the height gain effect of GnRHa treatment. Due to the ethical considerations, the researchers compared the final height with the initial PAH calculated by Bayley-Pinneau formula, with the absence of an untreated group.
Several studies have been carried out to evaluate final height after treatment with GnRHa. Almost all studies confirmed that final height was preserved or improved after treatment, with an average height gain ranging from 2 to 9.8 cm [16-19]. In our research, the PAH at the eventual time of the treatment of CPP girls was higher than their initial PAH by approximately 4.2 ± 0.6 cm. According to several studies, for females with CPP before 6 years old, GnRHa treatment results in an average gain in final height of 9 to 10 cm, whereas for those with treatment indication between 6 and 8 years, this therapy results in an average gain in adult height of 4 to 7 cm [4, 9, 20-23]. Until now, the indication of GnRHa therapy for CPP girls who visit the hospital after age 8 is a controversial issue because the average height gain in this age group is minimal, only 1 to 2 cm [24]. However, our study demonstrated that the predicted average height gain of females aged over 8 years was also remarkable, 3.2 ± 0.6 cm. According to the data of Asian girls with CPP, GnRHa therapy was still effective in the over 8 years old group, with a notable height gain of 3.79 cm in Le Ngoc Duy study, 4.8 cm in Baek et al. study and 3.71 cm in the study of Lee et al. [5, 25, 26].
Insufficient data exists for CPP males’ auxological outcomes with GnRHa therapy because this subject only occupies a small amount in the CPP population [9]. A Korean study showed that GnRHa treatment led to an increase of 4.1 cm in the final height in 85 boys with CPP [27]. Similarly, our study confirmed that the PAH after treatment increased by 2.9 cm compared to the initial PAH. However, male patients were only presented in small numbers in our research. Therefore, it is necessary to carry out other studies with large sample sizes to investigate the long-term outcome of boys with CPP.
Childhood overweight and obesity are risks of CPP in girls. Physicians have a concern about the influence of GnRHa therapy on BMI. Several studies have evaluated BMI outcomes in girls and boys with CPP. The majority of these investigations found no significant changes in BMI and fat mass during the period of treatment, but others have shown a mild decrease during the GnRHa regimen [16, 28-30]. According to data from the combined analysis, there was no significant change in BMI SDS after the GnRHa indication [4, 31]. Our study also confirmed that GnRHa treatment did not cause or worsen obesity. However, there is a need for further studies to appraise body composition and hormones correlated to fat metabolism in the future to have a more accurate analysis.
From our data, the side effects of GnRHa therapy were inconsiderable. The temporary flare of GnRH secretion can occur and result in transient vaginal bleeding in girls [30]. Other complaints such as menopausal symptoms or local reactions were not found in our study.
Another consideration of physicians involves the effects of GnRHa on BMD. Previous research has shown that BMD may decrease during the GnRHa regimen, although peak bone mass is not detrimentally influenced by GnRHa therapy [4, 16, 32]. According to data from a study, the author suggests that optimizing calcium and vitamin D intake may benefit BMD [33]. Although all BMD parameters and biochemical markers of bone turnover were within the normal range in our study, physicians should consider supplying calcium and vitamin D during GnRHa treatment in CPP patients who did not have appropriate growth velocity.
Our study had some limitations. Firstly, the absence of an untreated group due to ethical considerations has cast doubt on the effectiveness of treatment in this group. Secondly, we did not have data about the final height of these patients and only predicted the final height based on Baley-Pineau calculator. Finally, the subject population of boys was relatively small
Conclusion
In conclusion, our study confirmed that treatment for CPP with GnRHa is safe in the short term and might be effective in preserving predicted final height. Also, we suggest that the indication of GnRHa should be carefully considered in CPP females after age 8 because the height advancement prediction was still observed in this age group.