1. INTRODUCTION
In individuals with cancer, venous thromboembolism (VTE) including deep venous thrombosis (DVT) and pulmonary embolism (PE) is a highly prevalent complication. Approximately 4-20% of cancer patients will experience VTE and those with active cancer have a 5 to 7-fold increased risk of developing this [1]. A systematic review in Asian countries reported that the incidence of VTE in individuals with cancer was 1.85−9.88 per 1,000 person-years [2]. In particular, VTE is the second leading cause of death [3], causes frequent and prolonged hospitalization, and decreases the quality of life among individuals with cancer [4].
In Vietnam, cancer is the second leading cause of death in older adults (age ≥ 60 years based on the WHO’s definition) [5, 6]. Older adults with cancer have a significantly higher risk of developing VTE than younger ones because of several contributing factors, including older age [7], comorbidities [8], immobility [9], and frailty [10]. A study in Poland reported that older people with cancer experiencing PE had a hospital mortality rate six times higher and a long-term mortality rate three times higher than younger patients with cancer [11]. Moreover, 50% of deaths in older cancer patients with VTE occur within 3 months of the VTE diagnosis [12].
Vietnam is a rapidly aging country in Asia with approximately 12 million older people in 2019, and this number is projected to rise to approximately 30 million people in 2050 [13]. There are approximately 165,000 new cancer cases and 115,000 cancer deaths each year in Vietnam [14]. However, when we searched PubMed, Scopus, Web of Science, and Google Scholar using the keywords for thrombosis AND cancer AND Vietnam, no previous studies were available. Early recognition of the signs and symptoms of DVT and PE will help reduce related complications and mortality in older adults with cancer [15, 16]. Previous studies found early and appropriate treatment may reduce approximately 40% of mortality in PE patients [17, 18]. Therefore, we aimed to investigate the characteristics of VTE in older adults with cancer in the Geriatrics-Palliative Care Department of the University Medical Center Ho Chi Minh City, a university hospital.
2. MATERIALS AND METHOD
We conducted this study at the Geriatrics-Palliative Care Department of the University Medical Center Ho Chi Minh City, a university hospital.
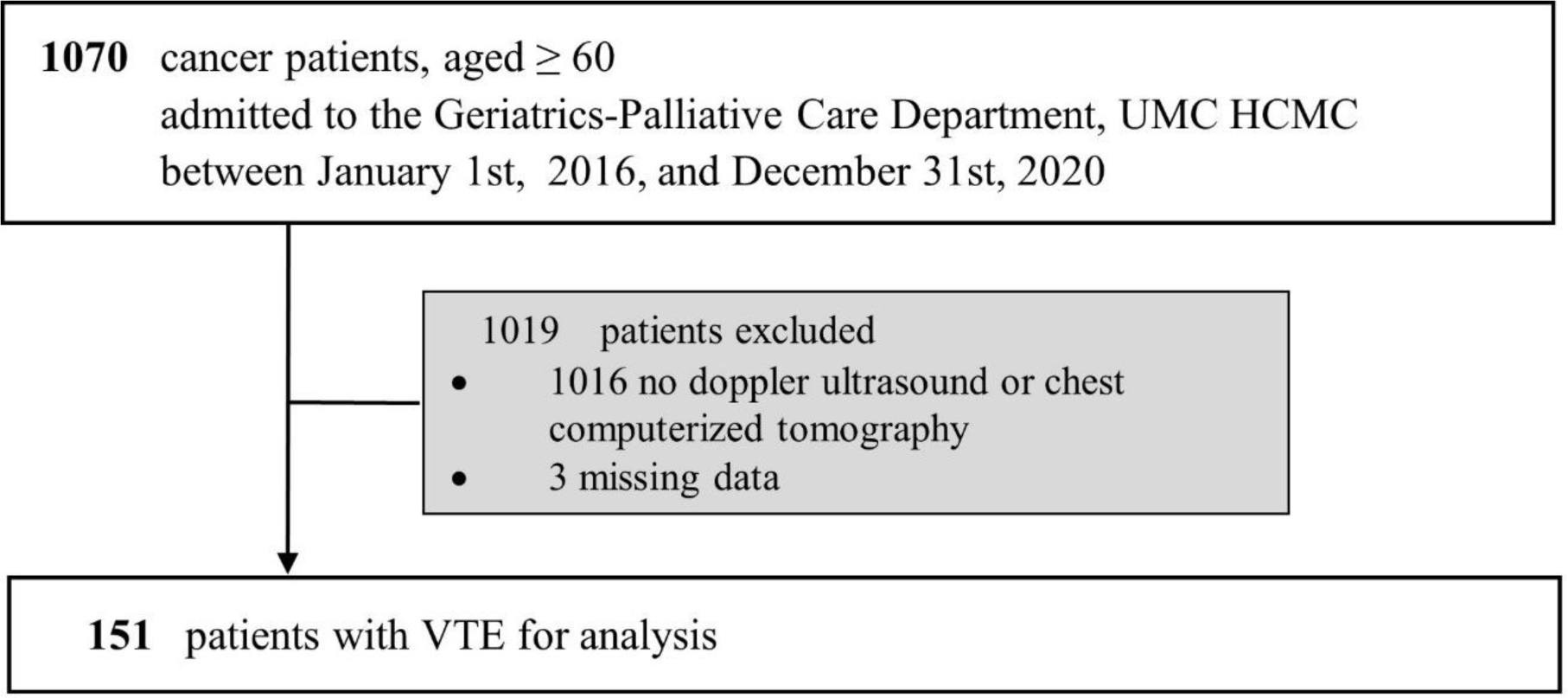
This was a retrospective study. We extracted data within 5 years, between January 1st, 2016, and December 31st, 2020. Inclusion criteria included: age ≥ 60 years, admission to the Geriatrics-Palliative Care Department during the study period, a new or confirmed diagnosis of cancer, and a new diagnosis of VTE. Exclusion criteria included: lacking any information related to demographics, laboratories, or treatments. Geriatricians collected data based on electronic medical records. Patients received no further follow-up after discharge.
Collected data included: (1) demographics including age, sex, and education level; (2) comorbidities; (3) type and stage of cancer; (4) admission diagnosis; (5) laboratory information such as X-rays, chest computerized tomography (CT) scans, ultrasound, and blood tests; (6) immobility; (7) length of hospital stay; and (8) treatment of VTE. Bleeding complications were followed up in patients receiving anticoagulants from the first date of use until the discharge date. Major bleeding complications were defined as fatal bleeding and/or overt bleeding in an area or organ such as bleeding that was intracranial, intraspinal, retroperitoneal, pericardial or intra-articular, or intramuscular with compartment syndrome and/or bleeding causing hemoglobin decrease of ≥ 2 g/dL or requiring transfusion of ≥ 2 units of red blood cells [19]. Immobility was defined as confinement to bed or chair at least 3 days before VTE diagnosis [20].
DVT and other types of venous thrombosis were confirmed using a doppler or abdominal ultrasound performed by specialists. PE was diagnosed using chest computerized tomography (CT) scans and the results were analyzed by diagnostic image specialists.
Patients aged 60-69 years were grouped as < 70 while those aged 70 years or older were grouped as ≥ 70 since previous studies explored that individuals aged ≥ 70 years tend to present atypical symptoms [21, 22].
Characteristics of the study participants were presented as mean ± standard deviation (SD) or median (interquartile range, IQR) for continuous variables, and as frequency (percentages) for categorical variables as appropriate. Differences in characteristics were evaluated using T-tests for continuous variables; and χ2 or Fisher exact tests for categorical variables by age groups.
Data analysis was carried out in Stata version 15.0 (StataCorp LP, College Station, Texas). Statistically significant levels are set at p values of 0.05.
3. RESULTS
A total of 1170 medical records with various types of cancer were reviewed during the study period, 1016 patients were excluded due to non-VTE diagnoses and 3 were excluded due to missing information. Consequently, data from 151 eligible patients were collected.
The general characteristics of the study population are presented in Table 1. The median age of patients who developed VTE was 67 (range 60 to 89). The highest rate of VTE was observed in patients with hepatocellular carcinoma (HCC) (50%), followed by those with lung cancer (14.6%). Approximately three-fourths of the individuals who developed VTE had stage IV cancer, and at the time of VTE diagnosis, nearly half of the patients (47%) were newly diagnosed with cancer. The results showed that 77.3% of patients with VTE had immobility. In our study, 92.1% of patients with VTE received cancer treatment, and the most frequent comorbidity among them was infection (39.7%, n = 60).
We found that portal vein thrombosis (48.3%) was the most common type of VTE, followed by PE (30.5%) and DVT (29.1%). Details of the VTE types are given in Figure 1.
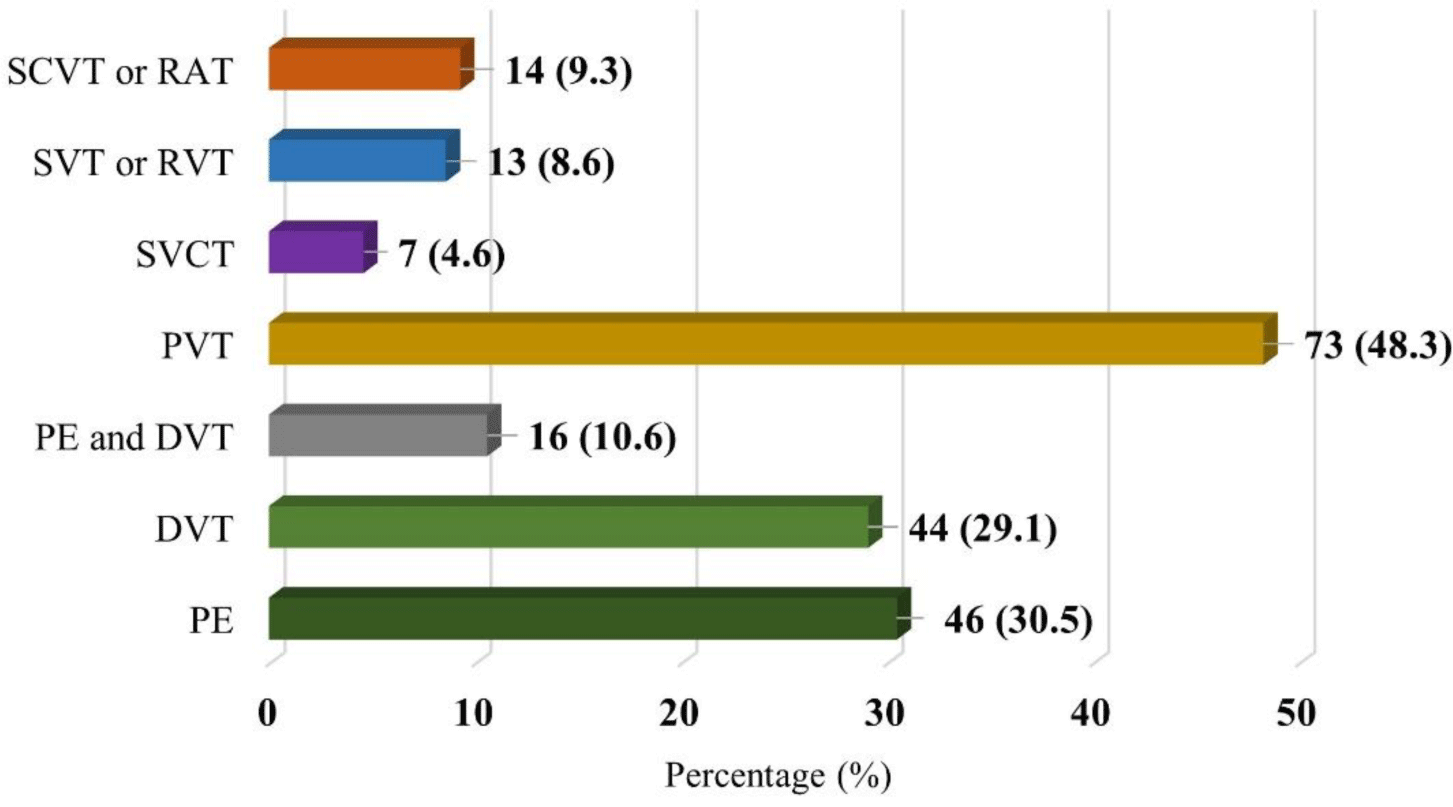
Table 2 presents the clinical characteristics of patients with DVT. Leg edema was the most frequent symptom (61.4%) in patients with DVT and thrombosis occurred more often in the left extremity than in the right. Meanwhile, dyspnea was the most frequent symptom (80.4%) in those with PE of all ages. For patients aged < 70 years, it appears that dyspnea and tachypnea were found at the same rate (Table 3).
Of the 151 patients who developed VTE, 51 (33.8%) patients were receiving anticoagulant therapy, 2 (1.3%) were receiving inferior vena cava filter therapy and 2 (1.3%) were receiving both therapies. The remainders did not receive VTE treatment due to contraindication, patients’ preference, or their diagnosis being asymptomatic portal vein thrombosis. Most of the patients (83%) received low molecular weight heparin (LMWH) (Enoxaparin 1mg/kg/12h) while 8 (15.2%) patients received unfractionated heparin (Heparin 80 UI/kg IV bolus, then continuous infusion of 18 UI/kg/h) followed by either warfarin or direct oral anticoagulant (DOAC) as the longer-term treatment. One patient was treated only with DOAC (Rivaroxaban 15 mg twice daily) for his VTE since he had atrial fibrillation and used daily DOAC. No major bleeding was recorded in this study (Table 4).
Table 5 presents the numbers of patients receiving treatment according to the sites of VTE. The patients with portal vein thrombosis had the lowest rate of receiving treatment.
4. DISCUSSION
This study aimed to identify the characteristics of VTE among older patients with cancer with the data collected within 5 years (2016-2020). We found that 151 older patients with cancer experienced VTE, and the three most frequent types were portal vein thrombosis, PE, and DVT. At the time of VTE diagnosis, approximately 50% of patients had newly diagnosed cancer. This could suggest that cancer screening should be performed in older adults with a new VTE diagnosis.
In our study, older patients with HCC accounted for half of the individuals with VTE. Hence, this is the reason why portal vein thrombosis was the most common type in our study (48.3%). Another study has shown that approximately 40% of patients with HCC developed portal vein thrombosis [23]. We explored that only 8.2% of patients with portal vein thrombosis received treatment since most of them were asymptomatic and incidentally diagnosed after routine abdominal ultrasounds. Indeed, there are no recent specific recommendations for asymptomatic chronic portal vein thrombosis treatment [24]. Therefore, more than 90% of individuals with portal vein thrombosis in our study did not receive treatment.
The results showed that approximately 60% of older patients with DVT presented with leg edema or leg pain. This finding is similar to the result of the author Piazza’s study, which found lower extremity symptoms were met in about 50% of older patients with DVT [25]. When patients get older, the symptoms will be more atypical due to geriatric syndromes [22]. Therefore, apart from leg edema, leg pain should also be considered a warning sign of DVT in older patients with cancer [26].
We also found that all individuals with PE presented with at least one of these symptoms and signs, including dyspnea, tachypnea, chest pain, or wheezing. These findings are in line with previous studies. Dyspnea and tachypnea are the most frequent manifestations in older patients with PE [27]. We revealed that the rate of dyspnea in older individuals with PE in our study is similar to the result of author Luca (80.4% vs 88%) [28], but the rate of tachypnea in our study is relatively higher than that in Luca’s study (71.7% vs 50%). Hence, a complaint of dyspnea in older patients with cancer should be considered a warning sign of PE and should not be overlooked in clinical practice.
Chest pain is another symptom of note, as our study observed the rate of chest pain in patients aged < 70 years were more than twice in those aged ≥ 70 years (73.1% vs 35%, p = 0.010). This is echoed by the author Ramos [29], who also reported that chest pain was reported in approximately 30% of patients aged ≥ 66 years with PE, which is significantly lower than in younger patients (53%).
In this study, 33.8% of patients with VTE received anticoagulant therapy, LMWH being the most common type. Although a higher age increases the risk of bleeding [30], our study recorded no major bleeding where patients received the recommended doses of anticoagulants. Previous studies showed that people aged 90 years or older are at a mildly increased risk of bleeding [30], and this age group only accounted for a small proportion of our study population.
However, our study also has several limitations. First, we did not conduct routine image screening to detect VTE in all cancer patients; thus, we have been underestimating the number of patients with cancer-related thrombosis. Second, we did not explore the risk factors of VTE in older patients with cancer. Third, this is a single-center study, which also excluded younger patients; hence, the data may not be representative of a more diverse population. However, to the best of our knowledge, this is the first study addressing the characteristics of VTE among older patients with cancer in Vietnam. It provides some implications in assisting physicians in the early recognition of DVT and PE in older adults with cancer, to help reduce the mortality rate and prolonged hospitalization among these patients. Moreover, our study will be the first step to developing a larger multicenter study, which will include a larger population and identify risk factors for developing VTE in Vietnamese patients.
Conclusion
VTE was highly common among older patients with stage IV cancer. At the time of VTE diagnosis, approximately 50% of cases had newly diagnosed cancer. Leg pain may be an indicator of DVT in older patients. More than 80% of patients with PE experienced dyspnea although five other symptoms and signs may be met, including tachypnea, chest pain, wheezing, rales, and hemoptysis. Patients aged ≥ 70 years were less likely to have dyspnea than patients aged < 70 years. No major bleeding was recorded in VTE patients receiving the recommended doses of anticoagulants.