1. INTRODUCTION
The World Health Organization estimated that the prevalence of chronic HBV infections is approximately 300 million people and each year, an estimated 600,000 people die due to hepatitis B and its complications [1]. Vietnam is an epidemiological area of hepatitis B and the prevalence of HBsAg (+) is 10.79%, which was one of the highest prevalence countries of HBV infections [2]. Currently, eight drugs are approved for circulation by the US Food and Drug Administration (FDA) for hepatitis B treatment [3, 4]. Tenofovir disopropyl fumarate (TDF) has been one of the FDA-approved medicines for treatment since August 2008. Tenofovir was effective in histological improvement, HBsAg loss, and anti-HBs seroconversion among hepatitis B patients. The effect was recognized in both Lamivudine and Adefovir resistance mutations. Additionally, patients only have to pay a moderate cost for TDF therapy. However, TDF still has many limitations including a low functional cure rate, many side effects especially affecting renal function, and the risk of drug resistance has not been ruled out [5]. Moreover, the HBeAg conversion rate is still not high. In a study among Vietnamese Hepatitis B patients, the HBV-DNA response (HBV DNA < 400 copies/ml) rate of Tenofovir monotherapy achieved 64.4% before the 12th, however, the HBeAg loss rate only accounts for 4% before the 12th. These results indicate the need to develop new antiviral therapy with higher efficacy for HBV treatment [6].
In 1988, the first clinical trial on the efficacy of Phyllanthus niruri in hepatitis B treatment was published by Thyagarajan SP et. al. [7]. Other medicinal herbs, such as Andrographis paniculata, Eclipta prostrata, and Taraxacum sinicum also have anti-inflammatory effects and can reduce liver cell necrosis to help improve liver enzymes. A randomized controlled trial in 2012 revealed the seroconversion efficiency of Phyllanthus Amari products (including Herba Phyllanthus Amari, Herba Andrographitis, Herba Taraxaci, and Herba Ecliptae) and Lamivudine combination therapy after 6 months accounts for 19% and is higher than that in Lamivudine monotherapy [8]. This is a combination of medicinal herbs from the long clinical experience of traditional medicine in the treatment of liver diseases [8, 9].
Another study among chronic hepatitis B patients with negative HBV DNA and positive HBeAg showed that HBeAg loss prevalence after 6 months treated with Phyllanthus Amari and Tenofovir combination therapy is 15.63% and is higher than that in Tenofovir monotherapy is 3.25% [9]. Although previous studies showed that a Phyllanthus1 Amari and TDF combination therapy is more efficacy than TDF monotherapy, however, HBeAg loss prevalence is lower than expected. The short-term follow-up of the trial may lead to that finding. Therefore, our study was conducted to compare the long-term efficacy of the serological, biological, and virological response of combination therapy of Phyllanthus Amari products (including Herba Phyllanthus Amari, Herba Andrographitis, Herba Taraxaci, and Herba Ecliptae) and TDF versus TDF monotherapy among chronic hepatitis B patients with positive HBeAg.
2. MATERIALS AND METHOD
Study design: A randomized controlled trial was performed from 2017 to 2020 at Le Van Thinh hospital in Ho Chi Minh City, Vietnam. Chronic hepatitis B patients who met the selection criteria were included. Randomize based on lotteries to assign the patients into 2 groups, including the combination therapy group (Com) and the monotherapy group (Mono).
Combination therapy: The combination therapy group was treated with Phyllanthus Amari products and Tenofovir. The monotherapy group was treated with Tenofovir and did not use Phyllanthus Amari products. Phyllanthus Amari products consist of Hearba Phyllanthus Amari 800 mg – Herba Andrographis 200 mg – Herba Ecliptae 200 mg – Herba Taraxaci 200 mg and are prepared into capsules. The Phyllanthus product of Khang Minh Pharmaceutical Corporation at D 19/37K Village Road 80, Hamlet 4, Vinh Loc B Commue, Binh Chanh District, Ho Chi Minh City. It is licensed by the Vietnam Ministry of Health and circulated nationwide. The Phyllanthus Amari product was orally treated at a dosage of 2 tablets x 3 times per day.
Monotherapy: Tenofovir (as Savi Tenofovir) 300 mg was orally treated at a dose of 300 mg/day (once daily).
Selection criteria: (1) adults aged from 18 to 60 years old, (2) patients who had chronic hepatitis B with HBeAg (+) and HBsAg (+) in over 6 months or HBsAg (+) combined IgM anti-HBc (-) if have not tested HBsAg before; HBeAg (+) and HBV DNA ≥ 105 copies/ml, ALT ≥ 80 UI / L (more than 2 times at usual index) [9] for at least 2 consecutive exams within 6 months.
Excluded criteria: (1) Patients who are co-infected with HCV, HIV, or other kinds of hepatitis diseases, alcoholic drinking. (2) Other acute and chronic conditions, such as heart failure, kidney failure, malignancy, advanced liver disease or decompensated cirrhosis, pregnant women, and lactating women.
Criteria for stopping research: During the study, were there symptoms of advanced liver disease severe flare-ups of hepatitis (eg, rapidly increasing jaundice, the sudden elevation of ALT more than 10) times normal value, the test has TQ% < 70%, albumin decrease < 35 g/l) or variable AFP and abdominal ultrasound carcinogenesis require a change in treatment.
The sample size is measured based on a formula comparing two ratios:
With α = 0.5 and β = 0.2; 0.2; the percentage of patients who lose HBeAg good after taking Tenofovir at 18 months is p1 = 0.15 [9]. The percentage of patients who lose HBeAg good after taking Tenofovir at 18 months is p2 = 0.32. From these data, the drawn minimum sample size for each group is 94 patients. The expected loss of sample rate is 10%; thus, it is necessary to select 209 patients to participate in the research.
Randomization was conducted to avoid bias and to blind the subjects and researchers. The randomness was organized at a 1: 1 ratio by lottery: (1) the combination group with odd numbers and (2) the monotherapy group with even numbers. Statistician (H.C.L) who was not involved in the recruitment and clinical assessment randomly assigned the participants to each group with the same probability. The independent statistician kept the generated randomization table; the file was collected from disclosure.
The combination group: The Phyllanthus Amari product (Phyllanthus Amari products conclusion of Herba Phyllanthus Amari, Herba Andrographitis, Herba Taraxaci, Herpa Ecliptae with radio 8:2:2:2) was orally treated at a dosage of 2 tablets x 3 times combine with Tenofovir (as Savi Tenofovir) 300 mg was orally treated at a dose of 300 mg/day (once daily).
The monotherapy group: Tenofovir (as Savi Tenofovir) 300 mg was orally treated at a dose of 300 mg/day (once daily).
The study included seven periods: a baseline of participants indicate study (T0), and a follow-up of the outcome after 1 month (T1), 2 months (T2), 3 months (T3), 6 months (T6), 9 months (T9), 18 months (T18). After the baseline period, the participants were randomly assigned to the combination group or the monotherapy group through restricted randomization. The method was based on the CONSORT statement (see more in Appendix, table Consort checklist)
HBsAg, HBeAg: Using automated immunoassay for HBsAg and HBeAg by Gemini machine (Germany). Conducted according to the HBsAg and HBeAg Immunoassay Technical Procedure automatically from the Department of Microbiology, Le Van Thinh Hospital.
HBV DNA: Quantify HBV DNA by Realtime PCR method with a detection threshold of 250 copies/mL. Conducted according to Taqman HBV DNA Quantitative Procedure of Laboratory Department, MEDIC LAB.
ALT, AST: Using enzyme kinetics method by Beckman Coulter AU-480 automatic biochemical analyzer. Conducted according to the Procedure of ALT (GPT) activity measurement test [blood] on biochemical blood AU480 of Department of Biochemistry - Hematology and Blood Transfusion, Le Van Thinh Hospital. The Normal value: ≤ 40UI/L.
Creatine serum: Quantification according to Jaffe method by ADIVA biochemical machine 1800. Conducted according to the Procedure of blood creatinine determination of the Laboratory Department, Le Van Thinh Hospital. The normal value is 0.7 – 1.2 mg/dl.
GGT: Using enzyme kinetics method by biochemical machine ADIVA 1800. Proceeded according to GGT quantification procedure of Laboratory Department, Le Van Thinh Hospital. The Normal value: ≤ 60UI/L.
In our study, three main outcomes were used, including serological responses (HBeAg loss), virological response (HBV DNA<250 copies/ml), and biochemical responses (ALT≤40UI/L). Biochemical responses and virological responses were assessed at 3, 6, 9, 12, 15, and 18 months after treatment, and ALT was assessed at 1, 2, 3, 6, 9, 12, 15, and 18 months after treatment.
The secondary outcomes included creatine serum, AST, and GGT. Creatine serum was assessed at 3, 6, 9, 12, 15, and 18 months after treatment. AST and GGT were assessed at 3, 6, 9, 12, 15, and 18 months after treatment to evaluate side effects.
Data were analyzed by STATA software version 14. Study showed peer-protocol analysis results. Overall lost to follow-up in our study is less than 5% and balance between groups, therefore peer-protocol did not different to intention to treat analysis (see more in Appendix, Table S2).
The comparability between groups was examined by comparing age, age group, and gender between combination therapy and monotherapy groups using the Mann-Withney test (age is non-normality distribution, Appendix, Table S1), Fisher’s exact test, and Fisher’s exact test, corresponding.
3. RESULTS
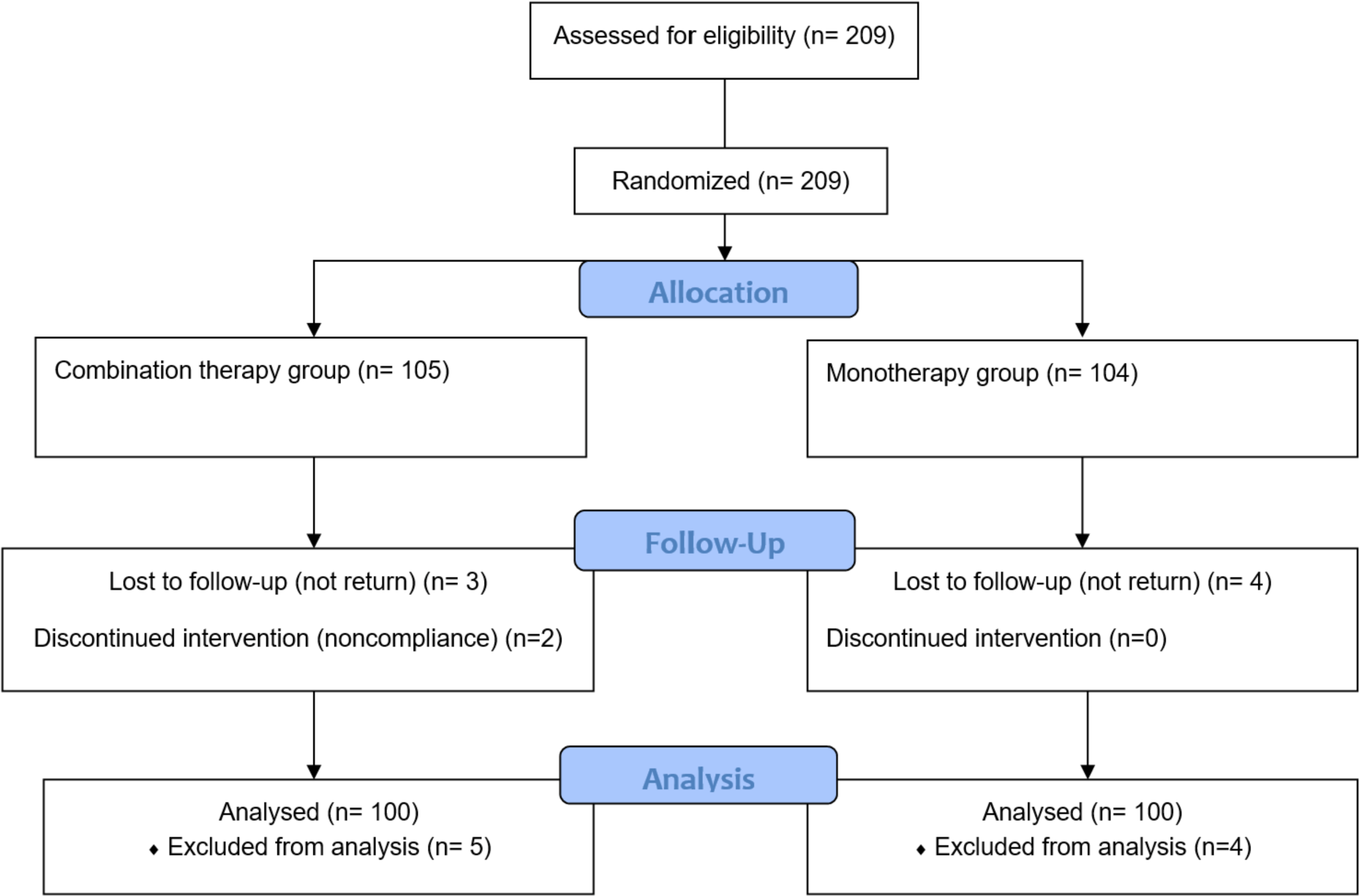
All 209 participants were screened for eligibility. Of these, nine met the exclusion criteria or withdrew consent (Figure 1).
In the research, age and gender showed a similarity in both groups. Males in the combination therapy group accounted for 56%, which was not statistically different compared to 45% in the monotherapy group with p = 0.157. The median ages in the combination therapy and monotherapy groups were 39 and 40.5, respectively (p = 0.646). The number of patients 40 years old or more in the two groups was not different significantly (p = 0.660). The Liver enzymes and HBV DNA is not statistically different in the two groups (Table 1).
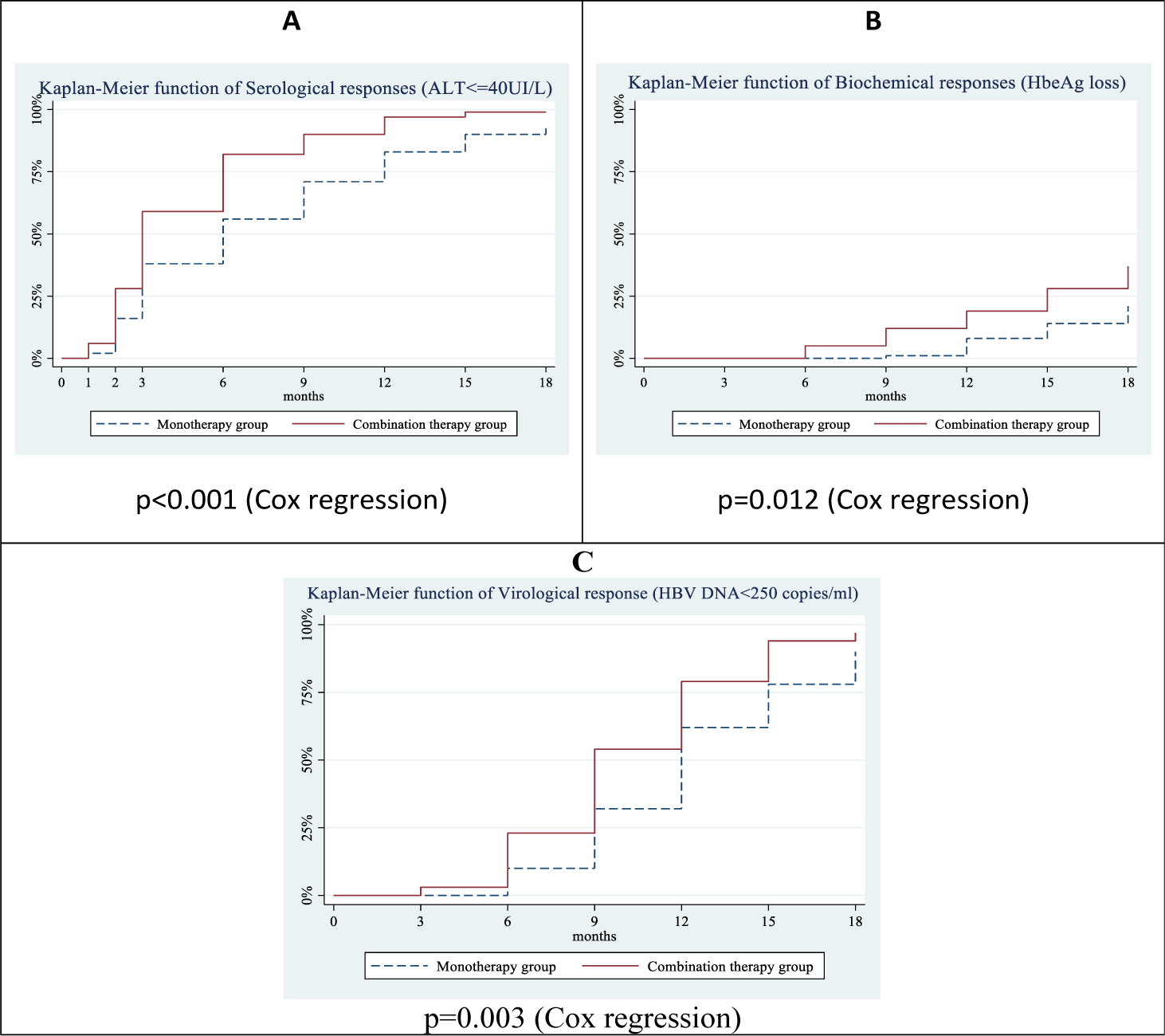
The combination therapy group started to achieve a serological response earlier than that the monotherapy group. Accordingly, the proportion of patients with ALT ≤40 UI/L increased steadily in both groups and these differences were statistically significant compared with baseline (T0). Response rates of ALT ≤40 UI / L at the 3rd, 6th, 9th, 12th, 15th, and 18th month in the combination therapy group were 59%, 81%, 88%, and 95%, 99%, 99%, respectively, higher than those of the monotherapy group, accounting for 33%, 52%, 67%, 76%, 86%, and 91%, respectively with a statistically significant difference (p<0.05). Statistically significant differences in serological response between the combination therapy versus monotherapy group were achieved from the 3rd (59% versus 33%, p=0.021) to the 18th month (99% versus 91%, p=0.029). Serological responses (HBeAg loss) did not occur in the first 3 months. Biochemical responses started to achieve in the 6th month in the combination therapy group and the 9th month in the monotherapy group. Statistically significant differences in biochemical responses between the combination therapy versus monotherapy group were achieved from the 9th (12% versus 1%, p=0.003) to the 18th month (35% versus 21%, p=0.040). The virological response in the combination therapy group started to achieve in the 3rd month and in the monotherapy group in 6th month. Statistically significant differences in virological response between the combination therapy versus monotherapy group were achieved from the 6th (23% versus 10%, p=0.021) to the 18th month (97% versus 88%, p=0.029) (Table 2)
In addition, the Kaplan-Meier function of treatment response showed a trend of earlier response in the combination therapy group than in the monotherapy group. Cox regressions indicated that the average probabilities of treatment responses are significantly different (p<0.05 for all study outcomes). (figure 2).
In both groups, blood creatinine tended to decrease slightly at some time. However, the trend of this change is not clear and the level of variation is no difference between the two groups of the combination compared with the group monotherapy (p>0.05). During the study period, creatinine was maintained between 0.87 mg/dl and 0.89 mg/dl in the combination group and in the range of 0.92 mg/dl to 0.95 mg/dl in the monotherapy group. No abnormal values of serum creatinine were observed during the study period rescue. The degree of change in creatinine did not differ between the two groups over time research.
AST concentration response of ≤ 40 UI/L in the combination group was observed early and was consistently higher than in the TDF monotherapy group alone during treatment (table 4).
The combination group or TDF monotherapy alone showed a significant reduction in GGT. GGT response rate ≤ 60 UI/L increased sharply from the first month of treatment in both study groups and maintained a decreasing trend throughout the treatment period (table 5).
Epigastric pain was observed in both groups with a low rate in the first month of the study of 7% in the The combination group and 2% in the TDF monotherapy. However, there was no statistical difference between the two study groups. After instructing the patient to The Phyllanthus Amari product containing hard capsules right after meals and supplementing with esomeprazole 20mg, 1 capsule 1 day before breakfast 30 minutes for 2 weeks, the patient had no symptoms of epigastric pain and did not notice later.
4. DISCUSSION
The study found that ALT levels decreased significantly in both groups right after the first month of treatment and continuously decreased steadily during 18 months of treatment compared to the baseline (T0). Comparing the serological response, we found that the response in the combination therapy group was superior to the monotherapy group in 18 months of follow-up (p<0.05). This is one of the most remarkable results for the treatment using Tenofovir and Phyllanthus product combination with a long-term follow-up than other studies (18 months versus 5-6 months in other studies) [8, 9]. The adverse effects of combination in this study were similar to those of previous studies to creatinine, AST, ALT, and GGT demonstrating that the combination of Tenofovir and Phyllanthus Amari product is safe to use in patients.
Moreover, we assessed the response by the level of change in each group at 8 points after the treatment compared to baseline (before the treatment). We witnessed that ALT reduction in the combination therapy group was significantly faster than in the monotherapy group during the first 3 months. From the 6th month on, there was no significant difference between the combined therapy group and the monotherapy group. The reason may be that starting from the 6th month onwards, the majority of cases had ALT levels back to normal (up to 81% in the combined group), so the decrease in this group was slower and less than in the previous stages. When comparing the results of the Tenofovir treated group in our study with other studies, the proportion of ALT normalization was nearly equal, and the combination therapy group showed a significantly higher rate (see Table 6). We, therefore, believe that the combination of TDF and Phyllanthus Amari product contribute to accelerating the normalization rate of ALT, thereby helping to minimize liver damage in the early stages of Tenofovir treatment. Possibly the mechanism underlying the Phyllanthus Amari product’s potent hepatoprotective effect is its ability to maintain glutathione in the reduced state by its antioxidative powers and inhibit lipid peroxidation [10].
| 3rd month | 6th month | 12th month | 18th month | |
|---|---|---|---|---|
| Dachuan Cai (2019) [14] | 44.59% | 66.88% | 81.94% | 84.97% |
| Rong-Yue Liang (2019) [15] | 52.73% | 71.95% | 82.93% | |
| Patrick Marcellin (2015) [17] | - | - | 63.1% | 72.7% |
| Our study (mono. group) | 33% | 52% | 76% | 91% |
| Our study (com. group) | 59% | 81% | 95% | 99% |
The study indicated that the response of HBV DNA and HBeAg was also better in the combination therapy group than in the Tenofovir monotherapy group. At 3rd month point, the HBV DNA index showed a positive immune response in both groups. Specifically, 82% of the combination therapy group decreased in HBV DNA concentration by more than 1 log of the number of copies/ml, and this one of more than 2 logs of the number of copies/ml after 3 months was 66%, a statistically significantly higher than 51% in the monotherapy group. They are similar to the results of GD Tong, X. Zhang, D. Zhou, D. et al. in 2014 which showed that HBV DNA concentration decreased by more than 2 log copies/ml at 22.2% (10/45 cases) in the combination therapy group (Tenofovir and Phyllanthus Amari product) higher than the control group was 5.0% (2/40 cases) (p = 0.023) [11].
In our study, the response in earlier stages of HBV DNA was lower than in other studies, this may be because our included sample was at a lower HBV DNA level. However, the outcome after 18 months showed that the rate of achieving HBV DNA below the detection threshold in the monotherapy group was similar, and the rate in the combination therapy group was significantly higher than the others (see Table 7). This result is consistent with previous in vitro studies, which reported on the inhibitory effect of HBV replication of the medicinal herbs of Phyllanthus Amari, Andrographis paniculata, and Taraxacum sinicum. The inhibitory effect of HBV replication in Phyllanthus Amari is also believed to be due to the inhibitory effect of DNA polymerase enzyme and reverse transcription enzyme due to its components nirtetralin B, and niranthin [12]. Therefore, we believe that combination therapy enhances the inhibition of HBV transcription compared with Tenofovir monotherapy.
| Writer | 3rd month | 6th month | 12th month | 18th month |
|---|---|---|---|---|
| Dachuan Cai (2019) [14] | 12.26% | 44.59% | 80.65% | 83.01% |
| Rong-Yue Liang (2019) [15] | 16.10% | 56.78% | 77.97% | - |
| Patrick Marcellin (2015) [17] | - | - | 60.05% | 71.9% |
| Our study (mono. group) | 0 | 10% | 62% | 88% |
| Our study (com. group) | 3% | 23% | 79% | 97% |
In our study, the response to loss HBeAg did not appear in two groups after 3 months (0%), On the other hand, Manas K. Behera (2020) reported a 10% response has loss HBeAg and Dachuan cai (2019) reported had 7.74% response loss HBeAg [13, 14]. But the response to the loss of HBeAg was reported earlier with a higher rate in the combination therapy group after 6 months (5%) however is lower than in previous studies [13-15]. It is 6 months earlier than in the monotherapy group. After 12 months, the combination therapy group had a significantly higher 19% HBeAg [16] seroconversion response compared with the Tenofovir monotherapy group, which accounted for 8%. When comparing results with other studies, the HBeAg loss rate of the monotherapy group in our study, although appearing later, the results after 18 months of treatment were similar to other studies (21%). However, the combination therapy group in our study showed a higher rate of response to HBeAg loss than the others after 18 months [17]. Except for Manas Kumar Behera et al indicated a much higher HBeAg loss rate than other studies (84%) [13], this could be explained because it was a retrospective cohort study, the HBeAg group sample size (+) included only 38 cases, which had a high risk of bias. (table 8)
| Writer | 3rd month | 6th month | 12th month | 18th month |
|---|---|---|---|---|
| Manas K. Behera (2020)[13] | 10% | 34% | 71% | 84% |
| Dachuan Cai (2019) [14] | 7.74% | 8.92% | 15.48% | 24.18% |
| Rong-Yue Liang (2019) [15] | - | 20.18% | - | - |
| Patrick Marcellin (2015) [17] | - | - | 8.3% | 14.7% |
| P. N. H. Tran (2013) [16] | - | - | 19.1% | - |
| Our study (mono. group) | 0% | 0% | 8% | 21% |
| Our study (com. group) | 0% | 5% | 19% | 35% |
Limitations of the study when it is not possible to not analyze the data using intention because of loss of samples and this can lead to errors selection bias, however, our study recorded a low sample loss rate (less than 5%) is less likely to influence the assessment results and 18-month follow-up study, the patients who lost follow-up from the first months may affect the evaluation of study results, so it is difficult to include and analyze.
The strengths of our study were to use a randomized controlled clinical trial with one treatment group and one control group in close supervision of researchers and monitoring of indicators continuously. In addition, per-protocol analysis was used in the combination with longitudinal examination with Multiple time points on therapy. Althought, after 18 months of follow-up, we evaluated these indicators at 9 points of time, more than other studies, and also described the disease progression through many treatment stages. Therefore, Cox regression was used to investigate the effect of several variables on the time a specified event takes to happen.
Conclusion
The combination of Tenofovir and the Phyllanthus Amari products has been shown more effective than using only Tenofovir for treatment in chronic hepatitis B patients with HBeAg-positive. The serological, biological, and virological responses in the combination therapy group in earlier than that in the monotherapy group.