1. INTRODUCTION
GLOBOCAN statistics in 2020 recorded approximately 2.3 million incidents of breast cancer, making it the most prev-alent cancer globally [1]. Advances in technology and disease monitoring have led to increase breast cancer diagnosis, while improved treatment options have contributed to higher rates of full recovery and extended survival [2,3]. The re-ported five-year survival rate for female patients with primary invasive breast cancer who underwent treatment (including surgery, radiotherapy, or systemic therapy) is 90% in high-income countries from the time of diagnosis [4,5]. Given the notably high survival rates for breast cancer patients, there is growing attention to the quality of life (QoL). Consequently, QoL has emerged as a critical focus in cancer clinical research and survival investigations, reflecting a broader concern for enhancing overall well-being beyond mere survival [2].
The positive impact of physical activity on the QoL of patients with breast cancer has been shown in previous studies [6–8]. The findings suggest that engaging in physical activity may improve breast cancer patients’ physical functioning, emotional well-being, and overall QoL. Moreover, the safety and practicality of physical activity for women with breast cancer were proven in previous studies, suggesting that adopting an active lifestyle could lead to improved clinical out-comes and QoL [6–8]. However, existing research has not adequately examined the effect of different types of physical activity on the QoL of breast cancer patients, and conclusive evidence is lacking. Therefore, we undertook a systematic review and meta-analysis to explore the relationship between various groups of physical activity and aspects of QoL in individuals diagnosed with breast cancer.
2. MATERIALS AND METHODS
We conducted a comprehensive search of the PubMed, Embase, Medline, Cochrane Library, and Google Scholar data-bases up to January 2023 to identify randomized controlled trials (RCTs) examining the effect of physical activity on QoL among breast cancer survivors. The search strategy incorporated a range of keywords related to QoL, physical ac-tivity, and breast cancer (Supplementary Table S1). This research adhered to the guidelines outlined in the Preferred Re-porting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020). Two reviewers independently per-formed the screening of papers, data extraction, and quality assessment. Any disagreements were resolved through dis-cussions involving a third author.
Our inclusion criteria were as follows: (1) peer-reviewed journal articles and grayscale databases of RCTs; (2) participants who were 18 years of age or older and diagnosed with breast cancer at any stage of the disease; (3) physical activity during the period between diagnosis and assessment of mental health; (4) the study evaluated the QoL outcomes of individuals with cancer; (5) there were no restrictions on race, sex, socioeconomic status, ethnicity, geographic area, or residence; (6) if a study investigated multiple types of cancer, only results specific to people with breast cancer were selected; (7) the publica-tion had to be in English, and the date of publishing was unrestricted. We excluded non-conforming research designs, studies lacking health-related quality of life (HRQoL) reporting, and studies with fewer than 20 participants.
Data extraction emcompassed several key elmenets from the included studies. These included research characteristics such as author’s name, year of publication, country, QoL assessment tool, intervention time, and follow-up durations. Participant characteristics covered population, sampling methodology, sample size, age, time since breast cancer diagno-sis, cancer stage, treatment method, study completion rate. Additionally, intervention specifics such as type, time, fre-quency, and intensity of physical activities were extracted. Finally the results of the QoL outcomes, including mean val-ues and corresponding 95% confidence interval (CI) were also recorded.
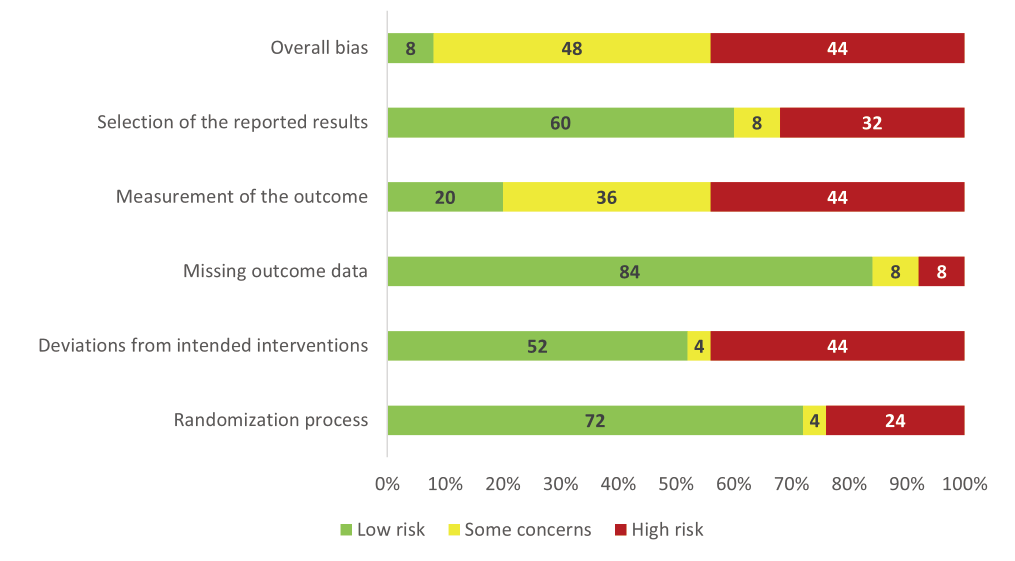
The evaluation of the studies incorporated in the analysis utilized the second version of the Cochrane risk-of-bias tool for randomized trials (RoB 2.0, Cochrane, London, UK). It includes five specific domains: (i) bias that arises as a result of the randomization process; (ii) bias resulting from deviations from intended interventions; (iii) bias that occurs due to missing outcome data; (iv) bias that occurs during the measurement of the outcome; and (v) bias that arises from the se-lection of the reported result. The tool assigns a rating to each bias domain, indicating whether the level of bias is low, unclear, or high. To assess overall bias using RoB 2.0, we evaluated each domain based on signaling questions and as-signed a judgment of “low risk,” “some concerns,” or “high risk.” If any domain has “some concerns” or “high risk,” the study will have the same overall judgment. If all domains have “low risk”, the study will have an overall “low risk” judgment for bias.
Following the guidelines provided by the World Health Organization (WHO) regarding physical activity and sedentary behavior for adults over 18 years of age with chronic medical conditions, we classified the studies selected into one of four groups of physical activity interventions: (1) Aerobic exercise: moderate-intensity for 150 minutes or high-intensity for 75 minutes weekly, with 0–1 muscle-strengthening session; (2) Muscle-strengthening: ≥2 sessions weekly, combined with moderate-intensity aerobic (<149 minutes) or high-intensity aerobic (<74 minutes); (3) Combination exercise: mod-erate-intensity aerobics for 150 minutes or high-intensity for 75 minutes weekly, plus ≥2 muscle-strengthening sessions; and (4) Other exercises: moderate-intensity aerobic (<149 minutes) or high-intensity aerobic (<74 minutes) weekly, with 0–1 muscle-strengthening session, or inclusion of endurance, flexibility, low-intensity exercise, or recovery rehabilitation [9]. The classification was determined by considering the characteristics of the intervention, including type, frequency, time, and level of intensity (Supplementary Table S2).
We calculated standardized mean differences (SMDs) separately for the intervention and control groups. Subsequently, a random-effects model was employed for the meta-analysis. The final analyses included separate assessments for HRQoL and change measures, with a detailed examination of each domain within every HRQoL measure. To facilitate analysis, we grouped similar domains of the EORTC QLQ-C30, FACT-B, and SF-36 that were similar were grouped, specifically overall QoL score, physical well-being, emotional well-being, functional well-being, and social well-being. We employed diverse measures to address the absence of SD and mean values in the meta-analysis. These included using standard errors, CI, t statistics, P values for mean differences, and imputation methods to estimate the missing values [10]. In assessing of heterogeneity in meta-analysis, we used either the Q statistic or the I2 index [11]. Heterogeneity was de-termined if the I2 value exceeded 50%. We conducted a subgroup analysis to examine the effects of the intervention in different subgroups, such as age, stage of disease, timing of treatment, mode of intervention, and type of control group, to ascertain if significant variances in the intervention effect exist.
We employed a visual examination of the funnel plots to evaluate the possibility of publication bias. Statistical analyses were performed using the Review Manager 5.4 software (Cochrane).
3. RESULTS
The screening and selection process is depicted in the flow diagram (Fig. 1). In the first stage of identification, 7,946 potential studies were retrieved from various databases and search engines. After removing 2,108 duplicate articles using EndNote X9 and Microsoft Excel, 5,838 studies underwent stage 2 (screening), which involved assessing titles and ab-stracts. Following this initial screening, 245 articles progressed to full-text screening to determine eligibility. Of these, 22 articles were excluded due to the unavailability of full texts. The remaining 223 articles excluded 198 studies based on the specified criteria for the final inclusion stage. We selected 25 studies suitable for systematic review; among them, 23 were included in the meta-analysis.
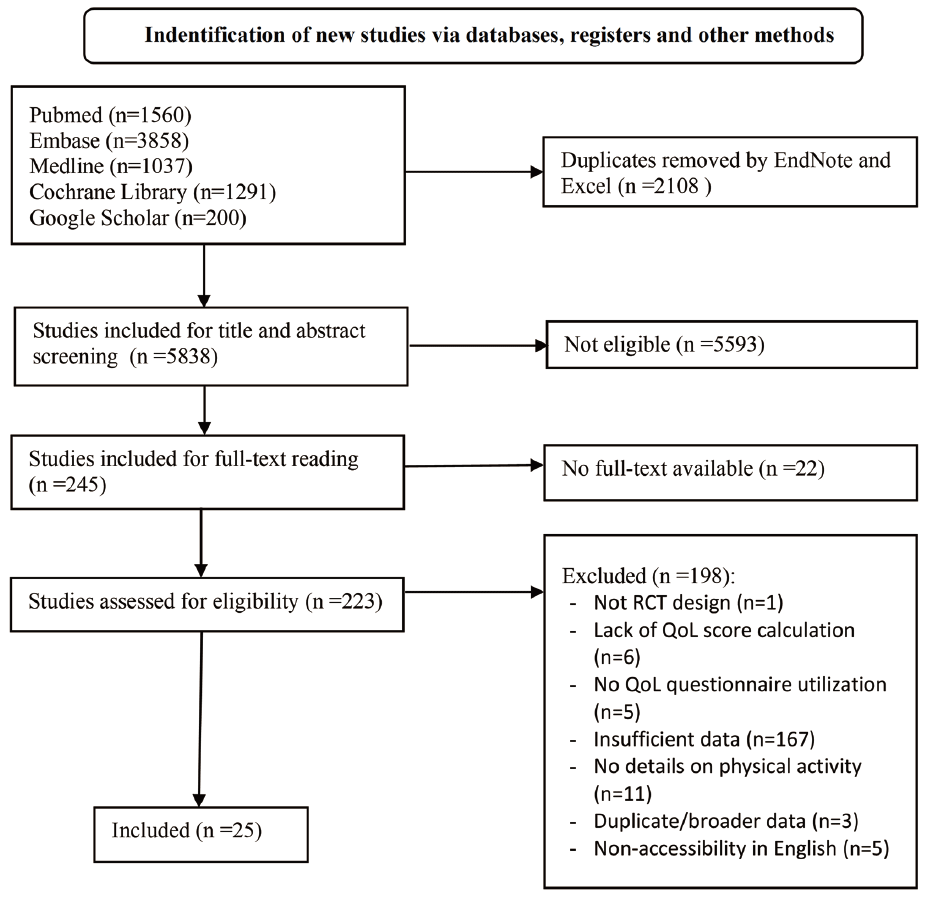
The article describes 25 studies conducted between 2003 and 2022, with 36% being from the USA and others from var-ious countries. Five QoL assessment tools were used during the studies, including EORTC QLQ C-30, EORTC QLQ BR-23, SF-36, FACT-G, and FACT-B. The sample sizes ranged from 40 to 220 individuals, all diagnosed with breast cancer at various stages. Our study included breast cancer patients (stages 0-III) undergoing treatment (chemotherapy, hormone therapy) or post-treatment (chemotherapy, radiotherapy, hormone therapy, surgery). Participants had characteristics such as overweight, obesity, breast cancer-related lymphedema, or joint pain. The participants were between 36 and 61 years old across all included studies. The time from breast cancer diagnosis to study participation ranges between 6 months and 9.5 years. Recruitment particularly took place in clinics, hospitals, and medical facilities through registration documents and doctor/therapist referrals with only one study recruiting participants through local newspaper advertising [12]. The studies involved physical activity intervention with control groups consisting of usual care or waiting lists. These studies encompass various exercise types, including strength training, aerobic exercises, interval training, yoga, martial arts, and more. The intensity ranges from low to high, and the duration of exercises varies, allowing exploration of their effects on breast cancer outcomes. Some studies use combination approaches, combining different exercise types, while others fo-cus on specific modalities. Details of patients’ characteristics and physical activity characteristics were clearly described in Table 1. Most studies had a single intervention group on physical activity, while others had two groups, and participant completion rates varied from 66% to 100%.
Regarding the overall bias, 48% of the studies were categorized as “some concerns,” 44% were “high risk,” and 8% were “low risk.” The measurement of outcome and deviations from intended interventions were the two domains where “high risk” bias accounted for the highest proportion (44%). Furthermore, many studies received a ‘low risk’ rating for bias, particularly in domains such as randomization, deviations from intended interventions, missing outcome data, and selection bias (Fig. 2 and Supplementary Table S3).
The findings of the study revealed that the aerobic exercise group demonstrated significantly elevated scores when compared to the control group in domains such as overall QoL score (SMD=0.45; 95% CI: 0.28 to 0.61; I2=20%), physi-cal well-being (SMD=0.52; 95% CI: 0.22 to 0.81; I2=73%), emotional well-being (SMD=0.35; 95% CI: 0.21 to 0.49; I2=0%), functional well-being (SMD=0.46; 95% CI: 0.19 to 0.72; I2=67%), and social well-being (SMD=0.27; 95% CI: 0.10 to 0.44; I2=21%) (Fig. 3). The group also experienced a decrease in fatigue score when measured with EORTC QLQ C-30 (SMD=–0.31; 95% CI: –0.56 to –0.06; I2=0%), and the breast cancer subscale mean score was significantly higher compared to the control group when measured with the FACT-B (SMD=0.33, 95% CI: 0.03 to 0.62, I2=52%). However, the change in mean QoL score between the two groups using SF-36 was not statistically significant in all domains (Sup-plementary Fig. S1).
The muscle-strengthening exercise group had a significantly higher mean score of QoL than the control group in do-mains such as overall QoL score (SMD=0.49; 95% CI: 0.12 to 0.87; I2=36%), physical well-being (SMD=0.62; 95% CI: 0.32 to 0.91; I2=0%), and social well-being (SMD=0.37; 95% CI: 0.06 to 0.68; I2=10%) (Fig. 3). There was also a statisti-cally significant increase in the mental health domain of SF-36 (SMD=0.50; 95% CI: 0.06 to 0.95; I2=0%). However, the change in the mean score of QoL was not statistically significant across all areas when comparing the muscle-strengthening group with the control group using EORTC QLQ C-30 (Supplementary Fig. S2).
The combination exercise group had notably better QoL outcomes than the control group across several domains, in-cluding physical (SMD=0.74; 95% CI: 0.40 to 1.08; I2=83%) and emotional well-being (SMD=0.51; 95% CI: 0.11 to 0.90; I2=87%), functional (SMD=0.63; 95% CI: 0.24 to 1.03; I2=87%) and social well-being (SMD=0.39; 95% CI: 0.09 to 0.69; I2=78%), and overall QoL score (SMD=0.73; 95% CI: 0.21 to 1.25; I2=93%) (Fig. 3). However, upon examina-tion of the EORTC QLQ C-30 data, it was observed that the combination exercise group exhibited more pronounced de-crease in QoL, displaying statistically significant differences, particularly in relation to fatigue (SMD=–0.61; 95% CI: –0.90 to –0.31; I2=0%) and pain (SMD=–0.58; 95% CI: –0.88 to –0.28; I2=0%). The study also found that the mental health domain of SF-36 was significantly improved in the combination exercise group compared to the control group (SMD=0.58; 95% CI: 0.01 to 1,16; I2=88%). Regarding the breast cancer subscale of FACT-B, there was no significant difference between the two groups (Supplementary Fig. S3).
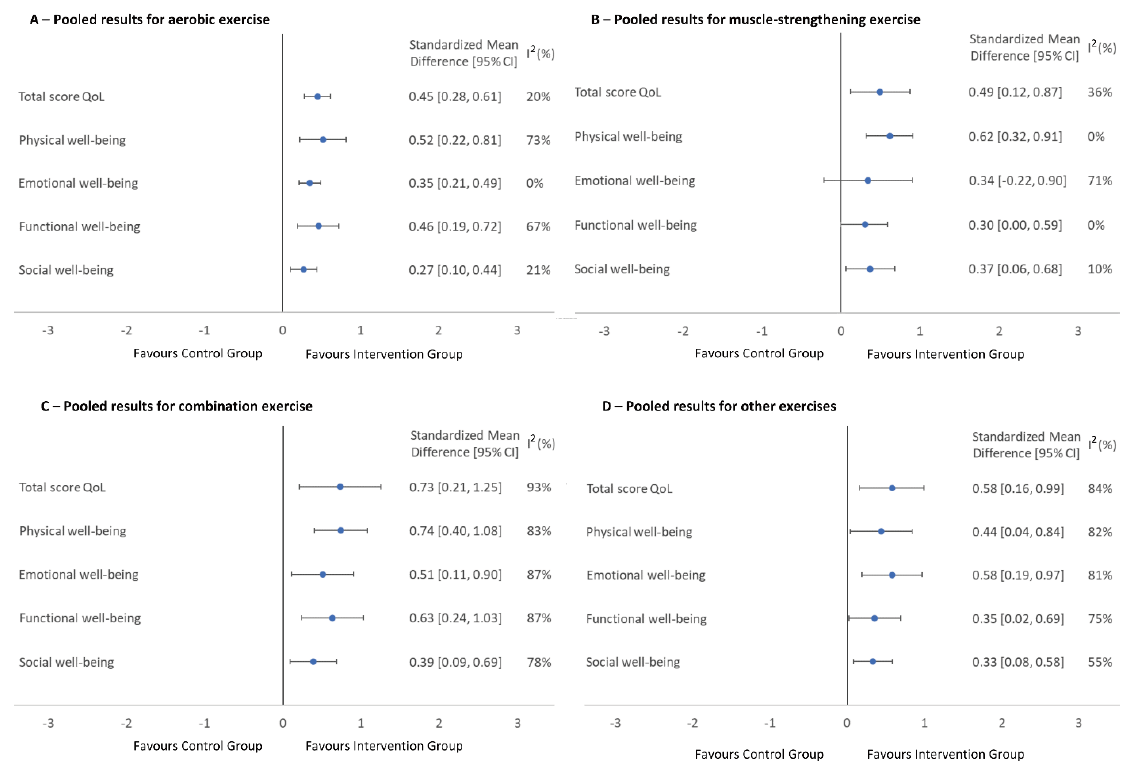
The QoL score significantly improved in the other exercises group compared to the control group across various do-mains, including physical (SMD=0.44; 95% CI: 0.04 to 0.84; I2=82%), emotional (SMD=0.58; 95% CI: 0.19 to 0.97; I2=81%), functional (SMD=0.35; 95% CI: 0.02 to 0.69; I2=75%), and social well-being (SMD=0.33; 95% CI: 0.08 to 0.58; I2=55%), and overall QoL score (SMD=0.58; 95% CI: 0.16 to 0.99; I2=84%) (Fig. 3). However, EORTC QLQ C-30 showed that the other exercise group had a more significant decrease in fatigue (SMD=–0.66; 95% CI: –1.18 to –0.15; I2=74%), pain (SMD=–0.98; 95% CI: –1.34 to –0.62; I2=46%), and constipation (SMD=–0.39; 95% CI: –0.70 to –0.09; I2=14%), but an increase in cognitive functioning (SMD=0.49; 95% CI: 0.06 to 0.92; I2=64%). There was a statistically significant increase in the breast cancer subscale of FACT-B in the other exercise groups (SMD=0.54; 95% CI: 0.29 to 0.78; I2=0%). The change in the QoL score of EORTC QLQ BR-23 was not significant in all the areas observed (Sup-plementary Fig. S4).
Supplementary Figs. S5, S6, S7, and S8 exhibited publication biases in specific domains, notably in physical well-being, dyspnea, insomnia, loss of appetite, diarrhea, future perspective, sexual enjoyment, and bodily pain. However, the distri-bution of research within the funnel graphs appeared symmetrical for the remaining domains [13].
4. DISCUSSION
The study explored how various forms of exercise affect the QoL among breast cancer patients. It revealed that Aerobic exercise, muscle-strengthening exercise, combination exercise, and other exercises regiments significantly improved QoL outcomes across different domains compared to the control group. It is noteworthy that the combination exercise group which showed notable enhancements in functional domains and reduction in symptoms such as fatigue and pain than the control group. Overall, exercise was found to be beneficial in improving QoL in breast cancer patients. Furthermore, these findings align with prior research, which has demonstrated that all three categories of exercise interventions - aero-bic, muscle-strengthening, and a combination of both - significantly improved the overall QoL among breast cancer pa-tients, with combined training linked to the most significant enhancement [37]. However, recent research suggested that while a combination of aerobic and muscle-strengthening exercises may lead to better QoL improvement compared to muscle-strengthening-only or aerobic-only exercises, the limited number of RCTs within each subgroup creates uncer-tainty about the measured effects, and yoga interventions showed no significant or meaningful effects on QoL measures, indicating their lower effectiveness when compared to other types of physical activity [6].
Due to the limited number of studies and the high degree of heterogeneity observed in our results, it was challenging to conduct subgroup analyses based on participant or intervention characteristics. Moreover, methodological heterogeneity was evident when assessing risk bias using Cochrane RoB 2, as the included studies exhibited varying degrees of risk bias. These variation led to inconsistencies and, consequently contributed to the observed heterogeneity in our study. More than 40% of the studies exhibited a high risk of bias in the outcome measure domain because the assessment results of the QoL relied on self-reporting by the study paritcipants. Given that the individuals involved were aware of their physical activity status, there were no objective measures available to corroborate the reported results. Given the general acknowl-edgement of the health benefits associated with physical activity, there is a potential for more favorable responses to out-come assessments, consequently presenting physical activity as more advantageous in research studies. The results of our research bias are also similar to those of the meta-analysis study by Aune [6].
Regarding publication bias, we discovered that funnel charts in various domains of the four QoL measures were poten-tially biased. The observed asymmetry in these plots, particularly when using the SMD as a measure, may lead to overes-timating the asymmetry [38]. This highlights the limited utility of this approach in evaluating publication bias, particular-ly when dealing with small sample sizes [38]. Therefore, caution is warranted in interpreting publication biases in our study.
Despite analyzing the data post-intervention, we were unable to find any significant change in the physical activity group concerning QoL scores in some areas. This could be attributed to having a limited number of studies (EORTC QLQ BR-23) or a short intervention period (12 months). Consequently, the impact of physical activity on these areas may not have been evident, or subjects could have started with high- QoL scores, making any significant improvement chal-lenging to achieve [14,15]. In the future, additional research should explore the long-term effects of physical activity on QoL in individuals with breast cancer and investigate how physical activity can help improve other aspects of QoL. Fur-thermore, researchers should also consider combining physical activity with other interventions to evaluate their joint effect on the QoL of patients with breast cancer.
5. CONCLUSION
Our findings suggest that aerobic exercise, muscle-strengthening exercise, combination exercise and other exercises im-proved the QoL in breast cancer individuals. There was no significant change in the physical activity group concerning QoL in various domains (physical, emotional, functional, and social well-being). Given the increasing number of breast cancer cases, incorporating physical activity as part of lifestyle counseling for breast cancer patients is essential. It can serve as a supportive intervention to facilitate lifestyle changes in individuals coping with breast cancer.