1. INTRODUCTION
Currently, many types of medicinal herbs are being used to treat diseases because the use of medicinal plants significantly reduces side effects compared to chemical drugs. In particular, medicinal herbs are used to support the treatment of immune-related diseases [1–3]. Periwinkle (Catharanthus roseus (L.) G. Don, Apocynaceae) is a medicinal herb that has been proven to have important pharmacological effects and is used in cancer treatment. In addition, periwinkle is also indicated in the treatment of acute lymphocytic leukemia [4,5]. In folk medicine, periwinkle is used to treat high blood pressure, diabetes, regulate menstruation, treat poor digestion and dysentery, treat hematuria, deworm and treat high fever [6–8]. Many studies have evaluated the cytotoxic effects of periwinkle on tumor cell lines to demonstrate its antitumor activity [9,10] as well as on peripheral blood mononuclear cells (PBMCs) [9]. However, there are not many studies on the immunomodulatory effects of periwinkle in Vietnam. Excessive response of the immune system is one of the factors that causes many immune-related diseases and worsens Coronavirus disease (COVID) infection [11]. Therefore, finding new natural remedies that have the ability to regulate the immune system can contribute to supporting the treatment and alleviating symptoms of diseases related to the immune system.
PBMCs plays an important role in the immune system. PBMCs include 70%–90% lymphocytes, 10%–20% monocytes, dendritic cells, and others. Lymphocytes are divided into T cells, B cells, and natural killer (NK) cells, of which T cells account for 70%–85%, B cells account for 5%–10%, and NK cells account for 5%–20%. PBMCs extracted from whole blood are widely used in toxicity studies [12]. Interleukin (IL)-1β is a proinflammatory cytokine produced by macrophages and plays an important role in the innate immune system and hematopoiesis. Interleukin-1β (IL-1β) activates T lymphocytes, B lymphocytes, neutrophils, and NK cells, and stimulates Th cells to produce IL-2 and plays an important role in the inflammatory process [13–15]. Therefore, when there is reduced production or overproduction of IL-1β, it seriously affects the function of immune cells as well as human health. Interleukin-6 (IL-6) is a proinflammatory cytokine that plays an important role in the adaptive immune response and has broad biological effects regulating many body processes. IL-6 is produced by T lymphocytes, B lymphocytes, and monocytes, which are rich in the fraction of PBMCs. IL-6 plays an important role in inflammation [16,17]. The decrease in IL-1β and IL–6 concentrations in the culture medium helps to evaluate the in vitro inhibitory effect of medicinal extracts on PBMCs proliferation which is a cellular fraction enriched in lymphocytes.
Currently, many models are used to study and evaluate the toxicity and effectiveness of medicinal herbs in the treatment of diseases related to the immune system in vitro or in vivo. The models are performed with the purpose of evaluating the effectiveness of natural medicinal herbs in proliferation and activation of immune cells, as well as inhibition and reduction of their activity [18]. The in vitro culture model of mononuclear immune cells isolated from human peripheral blood is one of the widely used models to test the proliferative or immunosuppressive activity of medicinal herbs. This study aimed to investigate the in vitro immunomodulatory activity of periwinkle leaf and root extracts on stimulated PBMC cells.
2. MATERIALS AND METHODS
Ficoll solution was from Cytiva HyClone. Roswell Park Memorial Institute (RPMI) – 1640 media, fetal bovine serum (FBS), penicillin/streptomycin antibiotic, phytohaemaglutinin – M (PHA), trypan blue solution and phosphate buffered saline (PBS) 1X were obtained from Gibco. Thiazolyl blue tetrazolium bromide (MTT) reagent was obtained from Sigma - Aldrich. Human IL-1β enzyme linked immunosorbent assay (ELISA) kit and IL-6 ELISA kit were obtained from Invitrogen. 96% ethanol was prepared for medicinal plant extraction. All reagent chemicals were under sterile conditions.
This study followed the CRIS guidelines [19] to improve the quality and transparency in reporting in vitro study.
This study evaluated the in vitro immunomodulatory effects of periwinkle roots and leaves on PBMCs cells isolated from human peripheral blood. Each experiment in this study was repeated twice. All in vitro assays in this study were performed on a 96-well plate with 1×106 PBMCs/well. Our previous study was found optimized blood volumes for these similar studies [1]. Therefore, the volume of human blood samples collected was similar to our previous study of five milliliters of whole blood samples from 10 healthy volunteers (5 men and 5 women) anticoagulated with lithium heparin. Blood samples were pooled prior to isolation of PBMC cells. Whole blood sample processing was conducted at aseptic conditions within 4 hours after blood collection. PBMCs were extracted from whole blood using Ficoll solution according to the manufacturer’s protocol with minor changes. In brief, 2.5 mL of heparin-anticoagulated whole blood was diluted 2-fold with 1X PBS in a sterile 15 mL falcon tube. After adding Ficoll solution to diluted blood in a ratio of 3:4, centrifuge the falcon tube at 400×g without braking for 30 minutes at 20°C. The plasma fraction was removed, PBMCs cells underlying the plasma layer were collected with a sterile pasteur pipette and placed in a sterilized 15 mL Falcon tube. PBMCs were washed twice with 6 mL of PBS. After centrifugation at 200×g for 10 min at 20°C, the supernatant was discarded, and the PBMCs pellet was resuspended in 250 µL of culture medium containing RPMI-1640, 10% FBS and 1% penicillin/streptomycin. PBMCs were stained with trypan blue and cell viability was evaluated using hemocytometer (viable cells over 95%). Then, cell density was corrected to 1×106 PBMCs/mL using cell medium before conducting the experiment.
This study evaluates the in vitro immunomodulatory effects of periwinkle roots and leaves on human PBMC cells.
The research medicinal sample was the leaf and root parts of periwinkle (C. roseus (L.) G. Don) provided by Hong Dai Viet in Phu Yen province, Vietnam and meets the criteria of Vietnam Pharmacopoeia V. Medicinal plant material was identified at Botany Department of Ho Chi Minh City University of Technology. Subsequently, medicinal plant material was washed to remove dust and mechanical impurities, dried in the air and then extracted by percolation.
PBMC cells were isolated from human peripheral blood. Blood donors who met the criteria for blood donation according to Circular 26/2013/TT-BYT issued on September 16, 2013, such as from 18 to 60 years old, minimum weight 42 kg for women and 45 kg for men, and did not use any drugs. The exclusion criteria for blood samples included missing sampling information, clotted blood, cracked or broken sample tubes, and anticoagulants other than lithium heparin.
One kilogram of dried medicinal materials (periwinkle leaves or roots) was crushed and sifted through a 2 mm diameter sieve, and then soaked with 96% ethanol in a ratio (1:10; kg/L) for 72 hours at room temperature. Periwinkle powder (leaves or roots) was extracted using the exhaustive extraction method with 96% ethanol at a flow rate of 3 mL/min at room temperature. The ethanol extracts were filtered to remove debris with filter paper and then concentrated with a rotary evaporator at 50°C to remove organic solvents, then left on a water bath at 60°C until all the water of hydration was removed. The total ethanol extract was stored away from light at a temperature of 4°C–8°C to prepare fractions or used for subsequent tests. The moisture content and dry extraction efficiency from raw periwinkle roots were 3.83% and 16.60%, respectively, and from raw periwinkle leaves were 4.02% and 23.52%, respectively (Table 1).
Fractionation of crude ethanol extract was carried out with different organic solvents to obtain n-hexane, chloroform, ethyl acetate and aqueous fractions. Dried medicinal plant extracts of different solvents and aqueous fraction were weighed and stored at −20°C until use. The fractionated extracts were stored away from light at a temperature of 4°C–8°C for use in subsequent tests.
The chemical composition of periwinkle leaf and root extracts was analysed for the presence of triterpenoids, alkaloids, coumarins, flavonoids, phenolic compounds, tannins, saponins, reducing sugars, starch and carotenoids following standard protocols [20]. Specifically, triterpenoids, alkaloids, and coumarins were identified using the Libermann–Burchard reagent, alkaloid reagent, and fluorescence test in an alkaline medium, respectively. Flavonoids, phenolic compounds, tannins and saponins were detected using reagent containing Mg2+ in concentrated HCl, 1% FeCl3 solution, 1% gelatin salt reagent and a foaming test in water, respectively. Reducing sugars, starch, organic acids and carotenoids were determined by testing with Fehling’s reagent, Lugol’s reagent, and the Carr-Price reagent, respectively.
Stock extract solutions were prepared by dissolving the dried crude extracts and fractions from periwinkle leaves or roots in dimethyl sulfoxide (DMSO) to obtain stock extract solutions at a concentration of 40 mg/mL. These stock extract solutions were filtered through a 0.22 μm filter and then stored at –20°C until use. Test extract solutions were prepared immediately before use by diluting the stock extract solutions with culture medium to achieve concentrations of 0.2; 2; 20; 100; 200; 400 ppm applied to cell cultures to investigate the inhibitory effect of the extracts on the proliferation of in vitro human PBMCs.
The cell culture procedure in the wells of a 96-well plate was performed as described in our previous research [1]. In the preparation step, 50 μL of PBMC suspension at a density of 1 ×106 PBMCs/mL were added into the wells of a 96-well plate and then incubated at 37°C, 5% CO2, 95±5% humidity for 2 hours to stabilize PBMCs. For a test sample, 50 µL of extracts at different concentrations were added to the cell wells to achieve a final concentration of extract in the wells of 0.1, 1, 10, 50, 100, and 200 ppm. For a negative control, cells were cultured with 50 µL of culture medium containing 3% PHA. For a blank sample, 50 µL of culture medium without PBMCs was added to 50 µL of extracts. For a blank control, only 100 µL of culture medium containing 3% PHA was plated in a well of the 96-well plate. After 24, 48, and 72 hours of incubation at 37°C, 5% CO2 and 90% humidity, 10 µL of a 5 mg/mL MTT solution was added to each well. Formazan crystals were formed after 4 hours of incubation and then dissolved with 200 µL of DMSO/NH3 solution. The optical density (OD) and the reference wavelength were read in triplicate on a multi well scanning spectrophotometer (ELISA reader) at 595 nm and 655 nm, respectively. The experiment was repeated twice. The inhibitory concentrations (ppm) where 50% of PBMCs inhibited (IC50) were determined. The proportion of living PBMC cells compared to the negatve control (%) was calculated by the following formula:
IL-1β was quantified using IL-1β Human ELISA Kit following the manufacturer’s instructions (Invitrogen). To investigate the inhibitory effect of extracts on in vitro IL-1β production by PBMCs, the crude extracts and fractions from periwinkle leaves or roots were diluted in culture medium to achieve at the concentrations of 0.2, 2, 20, 100, 200, and 400 ppm.
The cell culture procedure in the wells of a 96-well plate was performed as described in our previous research [1]. In the preparation step, 50 µL of PBMC suspension and 50 µL of culture medium containing 3% PHA were added in 96-well plate to achieve a final concentration of 1×106 cells/well. For a test sample, cells were cultured with 50 µL of extracts at different concentrations. For a negative control, cells were cultured in 50 µL of culture medium containing 3% PHA. After 48 hours of incubation at 37°C, 5% CO2 and 90% humidity, the medium in each well was collected and centrifuged at 1,020×g for 10 minutes. The supernatant was collected for IL-1β measurement or stored at –20°C until assay. IL-1β secretion by PBMCs in the cell culture medium was evaluated by immunoassay (IL-1β Human ELISA Kit, Invitrogen, Carlsbad, CA, USA). The OD of the final yellow product in the reaction medium was measured in triplicate using an ELISA reader at 450 nm. IL-1β concentrations in the samples were calculated based on the linear curve of the IL-1β standard. The experiment was repeated twice.
Mitogen-induced in vitro production of IL-6 was studied in PBMC cultures stimulated with PHA [21]. The investigation of the inhibitory effects of crude extracts and fractions from periwinkle leaves or roots on in vitro IL-6 production by PBMCs was performed similarly to the investigation of the effects of extracts on IL-1β.
Test extract solutions were prepared immediately before use by diluting the stock extract solutions with culture medium to achieve concentrations of 0.2, 2, 20, 100, 200, and 400 ppm applied on cell cultures to investigate the inhibitory effects of crude extracts and fractions from periwinkle leaves or roots on in vitro IL-6 production by PBMCs.
The cell culture procedure to determine the concentration of IL-6 in the samples was performed in a mannar similar to the experiment to determine IL-1β. For the assay, 50 µL of PBMC suspension and 50 µL of culture medium containing 3% PHA were added in 96-well plate to achieve a final concentration of 1×106 cells/well. For a test sample, cells were cultured with 50 µL of extracts at different concentrations. Thus, the final concentrations of the extract in the test wells were 0.1, 1, 10, 50, 100, 200 ppm. For a negative control, cells were cultured in 100 µL of culture medium with 3% PHA. At 48 hours of incubation at 37°C, 5% CO2 and 90% humidity, the medium in each well was collected and centrifuged at 1,020×g for 5 minutes. The supernatant was collected for IL-6 measurement or stored at –20°C until assay. IL-6 secretion by PBMCs in the cell culture medium was evaluated by immunoassay (IL-6 Human ELISA Kit, Invitrogen). The OD of the final formed yellow product in the reaction medium was measured in triplicate on ELISA reader at 450 nm. IL-6 concentrations in the samples were calculated based on the linear curve of the IL-6 standard. The experiment was repeated twice.
The leaves and roots of periwinkle were extracted with different organic solvents to obtain the crude ethanol extracts and fractions. These extracts were diluted in culture medium to create a series concentrations and then applied on PBMC cells. Extracts from medicinal samples were added to the 96-well plate with 1×106 PBMCs/well in order from low to high concentrations. Crude ethanol extracts were added to the 96-well plate first, followed by n-hexane, chloroform, ethyl acetate, and water fractions, respectively. The extract concentrations were marked on the 96-well plate to compare the stimulatory or inhibitory effects of periwinkle root and leaf extracts on PBMC proliferation, and IL-1β and IL-6 production by PMBC in duplicate after 24, 48, and 72 hours of incubation.
Statistical data were processed and analyzed using Microsoft Excel 2010. The concentration of extracts that inhibits 50% of in vitro proliferation of PBMCs (IC50 ppm) was determined using the non-linear regression algorithm of GraphPad Prism software version 10.00. Results were expressed as mean±SD or mean±SE. The t-test was used to compare the means of two groups of independent variables with a continuous normal distribution. Statistical analysis was considered significant if the p-value was <0.05.
3. RESULTS
Preliminary qualitative phytochemical tests showed that all crude ethanol extracts and fractions from periwinkle leaves and roots tested positive for alkaloids. The crude ethanol extracts, and EA and aqueous fractions from periwinkle leaves and roots contains mainly saponins and reducing sugars, while phenolic compounds were identified in the crude ethanol extracts and EA fractions. Crude ethanol extracts and H fractions from periwinkle roots tested positive for triterpenoid, but not in other extracts (Table 2).
The lower proportion of living PBMC cells in test samples containing periwinkle extracts compared to the control sample demonstrates that periwinkle extracts have a stronger inhibitory effect on PBMC proliferation. The proportion of living PBMC cells expressed as mean±SD, in test samples at different concentrations and control samples was presented in Figs. 1 and 2. After 24 hours of incubation, the majority of the extracts and fractions from periwinkle leaves showed an inhibitory effect on the proliferation of PBMCs cells compared to the control sample, except for CF fractions 10 ppm. The proportion of living PBMC cells ranged from 48.63% to 94.08% for EtOH extracts; 53.47% to 93.66% for H extracts; 27.08% to 102.78% for CF extracts; 36.81% to 85.14% for EA extracts; 32.64% to 88.89% for aqueous extracts. However, the inhibition of PBMCs proliferation did not depend on the extract concentration gradient, so it was not possible to determine the IC50 of each extract (Fig. 1A).
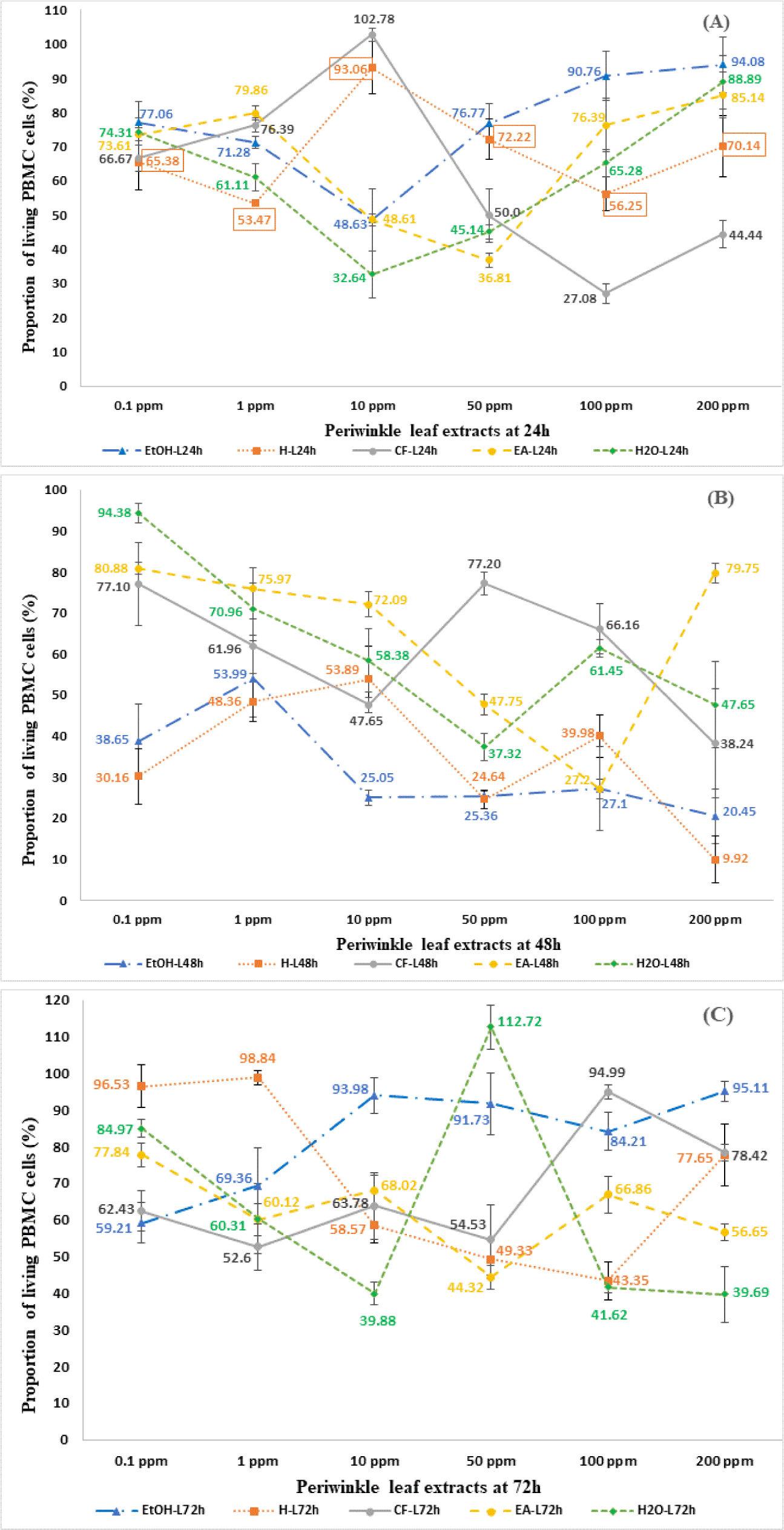
After 48 hours of exposure to crude and fractionated periwinkle leaf extracts, PBMC proliferation was strongly inhibited compared to control samples. The proportion of living PBMC cells ranged from 20.45% to 53.99% for EtOH extracts; 9.92% to 53.89% for H extracts; 23.24% to 77.20% for CF extracts; 27.20% to 80.88% for EA extracts; 37.32% to 94.38% for aqueous extracts. The IC50 concentration of the EtOH extract was determined at 6.1 ppm, while not for the other extracts because the inhibition of PBMC proliferation was independent of the concentration gradient of these extracts (Fig. 1B). After 72 hours of culture, the majority of periwinkle leaf extracts still showed inhibitory effects on PBMCs cells, except for aqueous fractions 10 ppm. The proportion of living PBMC cells ranged from 59.21% to 95.11% for EtOH extracts; 43.35% to 98.84% for H extracts; 52.60% to 94.99% for CF extracts; 44.32% to 77.84% for EA extracts; 39.69% to 112.72% for aqueous extracts 50 ppm. However, the cells showed recovery compared to the 48-hour time point (Fig. 1C).
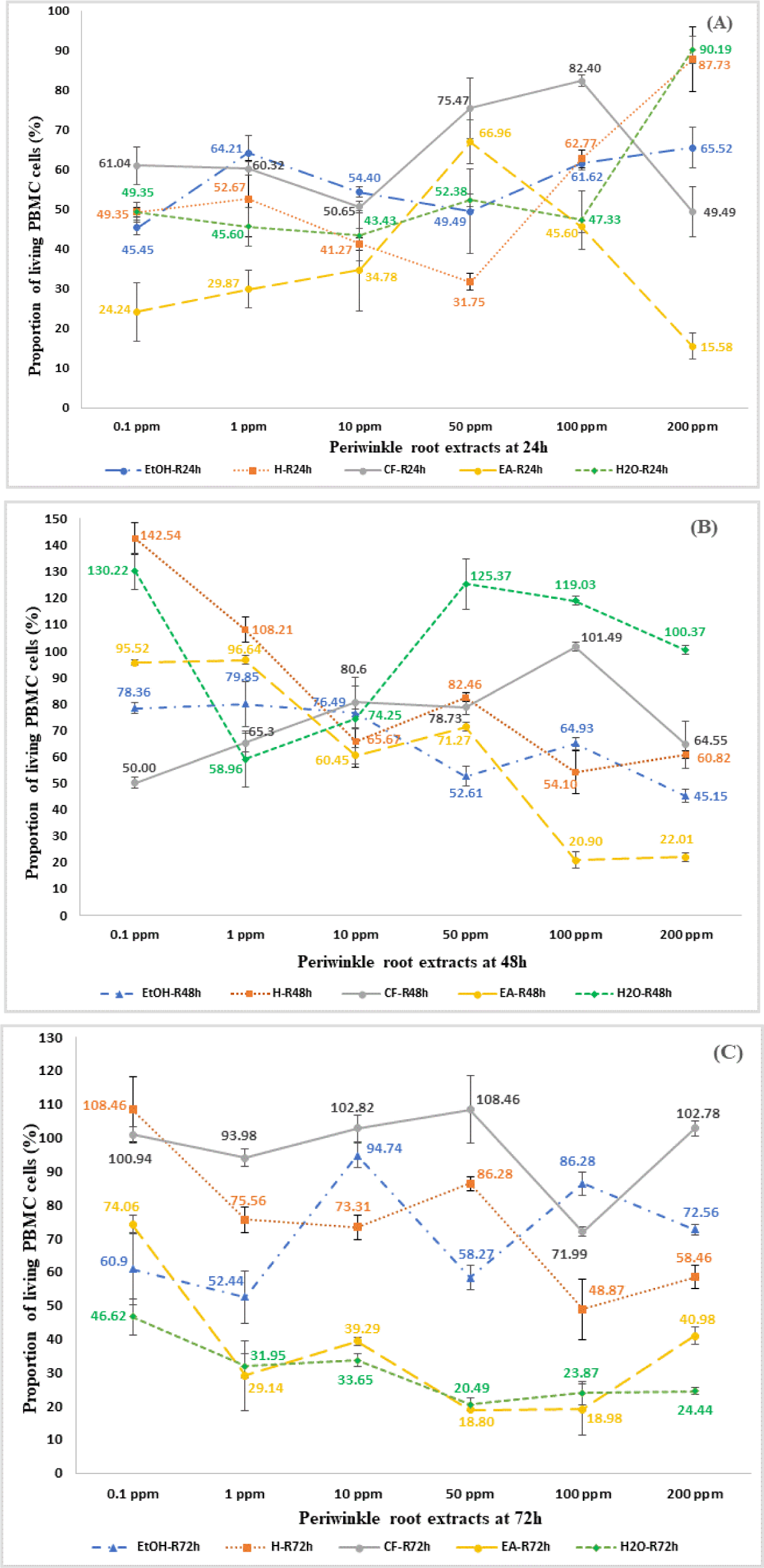
After 24 hours of incubation, the majority of the the EtOH extract and fractions from periwinkle roots showed an inhibitory effect on the proliferation of PBMCs cells compared to the control sample. The proportion of living PBMC cells ranged from 45.45% to 66.52% for EtOH extracts; 31.75% to 87.73% for H extracts; 49.49% to 82.40% for CF extracts; 15.58% to 66.96% for EA extracts; 43.43% to 90.19% for aqueous extracts. However, the inhibition of PBMCs proliferation did not depend on the extract concentration gradient, so it was not possible to determine the IC50 of each extract (Fig. 2A). After 48 hours of exposure to crude and fractionated extracts, PBMCs cell proliferation was inhibited compared to control samples, except for H fractions 0.1 ppm and CF fractions 100 ppm. The proportion of living PBMC cells ranged from 45.15%–79.85% for EtOH extracts; 54.10%–142.54% for H extracts; 50.00%–101.49% for CF extracts; 20.90%–95.92% for EA extracts; 58.96%–99.03% for aqueous extracts. However, the inhibition of PBMCs proliferation did not depend on the extract concentration gradient except for EtOH extracts. The IC50 concentration of the EtOH extract was determined to be 51.17 ppm (Fig. 2B). After 72 hours of culture, CF fractions did not show a clear inhibitory effect on cell proliferation, but the EA and aqueous fractions exhibited the strongest proliferation inhibitory effect. Recovery of PBMCs was observed in samples incubated with the crude extract and n-Hexane fractions. The proportion of living PBMC cells ranged from 52.44% to 94.74% for EtOH extracts; 48.87% to 108.46% for H extracts; 71.99% to 102.82% for CF extracts; 18.80% to 74.06% for EA extracts; 20.49% to 46.62% for aqueous extracts (Fig. 2C).
MTT test results showed that the percentage of live PBMCs cells compared to control samples at 72 hours recovered. The crude extracts and fractions from periwinkle leaves and roots showed the strongest and most stable effects on PBMC proliferation within 48 hours. Therefore, the impact of total and fractionated extracts of periwinkle roots and leaves on the production of IL-1β and IL-6 by PBMCs cells after 48 hours of culture was evaluated.
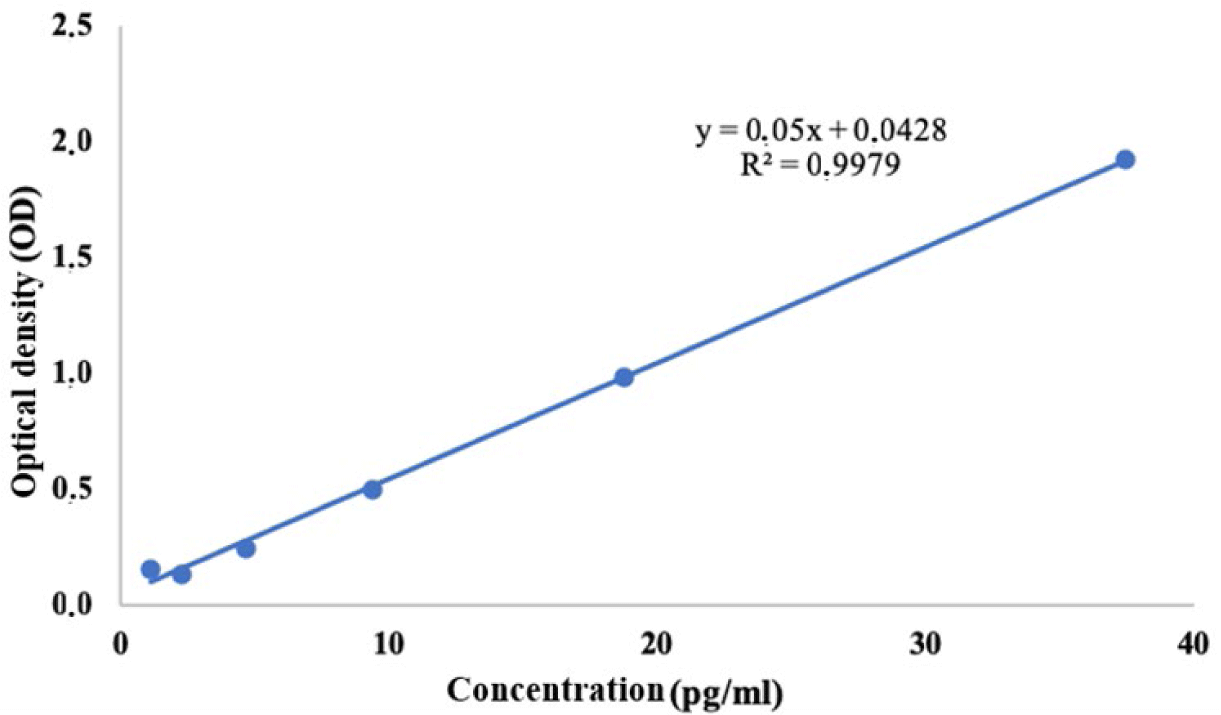
IL-1β concentrations in testìng and control samples were calculated based on the linear equation y = 0.05x + 0.0428 (R2 = 0.9979), where, y was the OD, x is the IL-1β concentration (pg/mL) (Fig. 3). Periwinkle extracts had an inhibitory effect on IL-1β secretion by PBMCs if the IL-1β concentration determined in the test samples was lower than the concentration in the control samples.
The results of each concentration were normally distributed and expressed as mean±SE. The t-test was used to compare the difference in IL-1β concentration between test samples and control samples. Most crude and fractional extracts from periwinkle leaves at different concentrations exhibited a significantly inhibitory effect on IL-1β production by PBMCs compared to the control (p<0.01), in which chloroform leaf fraction (1–200 ppm) showed the strongest inhibition and followed by EA fractions (Table 3).
The crude and fractional extracts from periwinkle roots at different concentrations also demonstrated an inhibitory effect on IL-1β production by PBMCs. However, the EA fraction (100–200 ppm) showed stronger inhibition than CF fractions. The aqueous root fraction roots inhibited IL-1β production with IC50 value of 38.46 ppm (Table 3).
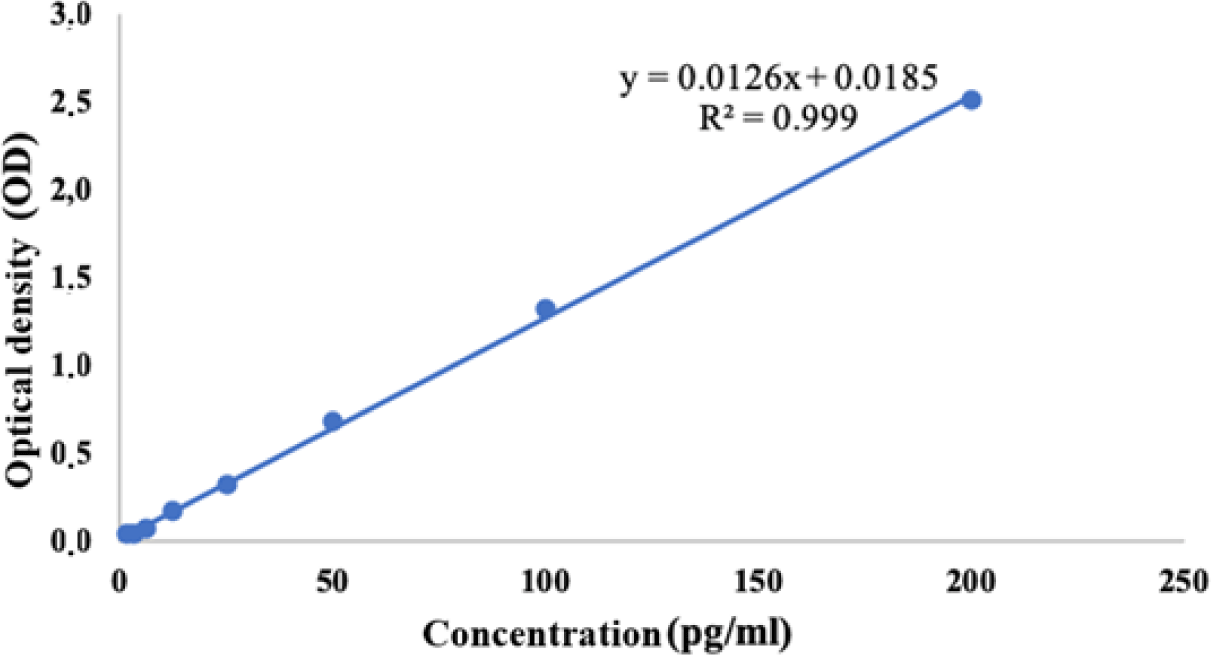
IL-6 concentrations in test and control samples were calculated based on the linear equation y = 0.0126x + 0.0185 (R2 = 0.999), where, y represents the OD, x represents the IL-6 concentration (pg/mL) (Fig. 4). Periwinkle extract exhibited an inhibitory effect on IL-6 secretion by PBMCs if the IL-6 concentration determined in the test samples was lower than the concentration in the control samples.
Most crude and fractional extracts from periwinkle leaves at different concentrations exhibited an insignificantly inhibitory effect on IL-6 secretion by PBMCs compared to the control. Both leaf and root CF fractions showed the inhibition on IL-6 secretion by PBMCs stronger than other fractions. CF leaf fractions strongly inhibited IL-6 production secreted by PBMCs with IC50 value of 41.37 ppm (Table 4).
4. DISCUSSION
Periwinkle (C. roseus (L.) G. DON, Apocynaceae) is a widely used medicinal herb worldwide. Many studies have shown that the 96% ethanol extract of periwinkle exhibites antioxidant, anti-cancer, and anti-diabetic effects [22–24]. Therefore, this study used 96% ethanol for the total extraction. Preliminary results of the chemical composition of the extracts showed that the whole leaf extract contained alkaloid compounds, phenolic compounds, saponins and reducing sugars. The chemical composition of whole root extract is more diverse than that of whole leaf extract, including alkaloids, phenolic compounds, saponins, triterpenoids, coumarins and reducing sugars. A previous study demonstrated that the ethanol extract of periwinkle leaves contains alkaloids, flavonoids, saponins, phenolic compounds, anthocyanins, steroids, glycosides and tannins [25]. In addition, another study only found alkaloids and saponins in the ethanol extract of periwinkle roots [26]. This difference may be due to differences in location and time of collection.
PBMCs are primary cell lines and do not have the ability to divide indefinitely [27]. Studies on the effects of medicinal extracts on PBMCs were conducted for a maximum of 5 days. Therefore, experiments on PBMCs in this study were investigated at three time points: 24 hours, 48 hours, and 72 hours. Results of investigating the effects of periwinkle leaf extract on PBMCs cells using the MTT assay in this study showed that, in the first 24 hours, leaf crude extract and fractions showed inhibitory effects on PBMCs. At 48 hours, the inhibitory effect on PBMCs cells of whole leaf crude extract and fractions was more evident. Leaf crude extract showed strong concentration-dependent inhibition of PBMCs with an IC50 of 6.1 ppm. At 72 hours, the extracts still showed inhibitory effects on PBMCs cells, but this effect was no longer as strong as at 48 hours. As for periwinkle root extracts, in the first 24 hours, the total extracts and fractional extracts of periwinkle roots showed inhibitory effects on PBMCs cells. At 48 hours, the inhibitory effect on PBMCs cells was still shown but the cells began to recover. Root crude extracts and EA fractions inhibited PBMCs cells concentration-dependently with IC50 of 51.17 ppm for root crude extract and IC50 of 76.52 ppm for EA fractions. At 72 hours, the CF fraction of periwinkle roots did not show a clear inhibitory effect on PBMC cells. This shows that the effect of CF fraction is a short-term effect, lasting only up to 48 hours. Research by Ahmad NH et al showed that the aqueous extract of periwinkle leaves had the effect of increasing PBMCs cell proliferation at a concentration of 1,000 ppm at 24 hours, however at 72 hours PBMCs cells were slightly inhibited at low concentrations of extracts [9]. PBMC cells in the study Ahmad NH et al were treated with the aqueous extract from periwinkle leaves at 1,000, 500, 250, 125, 62.5, 31.25, 15.63, 7.81, or 3.91 ppm for 72, 48, or 24 hours [9]. In our study, PBMC cells were treated with the crude extract and fractions from periwinkle leaves and roots at 200, 100, 50, 10, 1, and 0.1 ppm. Therefore, the differences between studies on the inhibitory effect on PBMC proliferation may be due to differences in extraction solvents, chemical compounds of the medicinal extract fractions, and PBMC cell culture conditions such as extract concentrations in test samples. In addition, chemical interactions between compounds in the crude extract and fractions from periwinkle leaves and roots at different concentrations may cause the inhibitory effects of crude extract and fractions from periwinkle leaves and roots on PBMC cell proliferation as compared to the control [28].
Preliminary qualitative results showed that periwinkle leaf and root extracts all contain alkaloids. Some studies have shown that alkaloids exhibited inhibitory effect on PBMCs cell proliferation [29,30]. In addition, other organic compounds such as saponins, triterpenes, and phenolic compounds have anti-inflammatory and immunomodulatory properties [31,32]. Therefore, extracts from the leaves and roots of periwinkle exhibited inhibitory effect on PBMCs cells, which are immune cells that play a crucial role in immunity [23]. This is consistent with previous studies showing that periwinkle has cytotoxic and immunomodulatory effects [9,33,34].
IL-1β is a proinflammatory cytokine produced by monocytes and macrophages and plays an important role in the innate immune system and hematopoiesis. IL-1β activates T lymphocytes, B lymphocytes, neutrophils, and NK cells, and stimulates Th cells to produce IL-2 and plays an important role in the inflammatory process [13–15]. IL-6 is a proinflammatory cytokine that plays an important role in the adaptive immune response and exerts broad biological effects regulating many body processes. IL-6 plays a significant role in inflammation [16,17]. During the COVID-19 pandemic, cytokine release syndrome is one of the main causes of deterioration in patients infected with the COVID-19 virus [11]. Therefore, inhibiting IL-1β and IL-6 overexpression contributes to the treatment and relief of symptoms in patients with COVID-19 infection as well as other autoimmune diseases. In our study, most periwinkle leaf and root extracts at different concentrations strongly reduced IL-1β production by PBMCs after 48 hours. The CF fraction of periwinkle leaves was found to most strongly inhibit IL-1β production by PBMCs with the amount of IL-1β below the detection limit at the tested concentrations. According to previous study, periwinkle alkaloids have an inhibitory effect on the formation of inflammatory mediators such as TNF-α, IL-1β [34]. In addition, triterpenes and saponins have also been reported to reduce the production of IL-1β and IL-6 by PBMCs [35,36]. Therefore, the inhibitory effect of PBMCs on IL-1β secretion may result from a synergistic effect between compounds in periwinkle leaf and root extracts. In this study, the total root extract, EA fractions, and aqueous fractions of periwinkle roots and leaves did not exhibit an inhibitory effect on IL-6 production by PBMCs at 48 hours. However, the CF fraction of periwinkle leaves demonstrated the most concentration-dependent inhibitory effect on IL-6 production of PBMCs. An in vivo study demonstrated that periwinkle alkaloids inhibit IL-6 and IL-1β in mouse peripheral mononuclear cells [37]. Another study showed that periwinkle leaf extract reduced IL-6 levels in rats after 6 weeks of administration [38]. Thus, the inhibitory effect on IL-6 production of PBMCs cells may be caused by alkaloids.
The results in this study showed that inhibition of PBMC proliferation in culture media with extracts may be associated with the inhibition of IL-1β and IL-6 production by PBMCs. A limitation of this study was that the purified substances in the fractions were not isolated, and their structures were not determined. As a result, it was not possible to identify which group of compounds exerts the immunoregulatory effect on PBMCs. Therefore, further studies on the chemical composition and structure of the compounds in the extracts are needed to better understand the effects of periwinkle leaf and root extracts on PBMCs.
5. CONCLUSION
The extracts from the leaves and roots of C. roseus exhibited inhibitory effects on PBMC proliferation and interleukin secretion from PBMCs. Both chloroform and ethyl acetate fractions of leaves and roots demonstrated the strongest inhibitory effects on IL-1β and IL-6 secretion by PBMCs. Extracts of C. roseus may be considered for the treatment of autoimmune diseases.