1. INTRODUCTION
According to a report by the Vietnam National Heart Association, there are approximately 25% of the population currently suffers from heart disease and high blood pressure. Hypertension and heart failure were among the leading causes of morbidity and mortality in Vietnam [1]. One of the most commonly used methods to diagnose and treat cardiovascular disease involves the use of fluoroscopic technique. Fluoroscopy enables real-time imaging to guide clinicians in performing both diagnostic and treatment procedures through tiny cuts on the patient’s body. With lower cost, reduced risk, and less pain compared to traditional surgical methods, fluoroscopic techniques are increasingly being used in clinical practice. However, one significant disadvantage of this method is that the medical team performing the procedures must work inside the X-ray room, continuously exposed to radiation. Depending on the complexity of each procedure, the time required can range from several minutes to hours. The frequency-weighted effective dose for interventional radiology was approximately 15 mSv per procedure, with the most common procedure - percutaneous transluminal coronary angioplasty - delivering an effective dose of up to 20 mSv per procedure [2]. Several studies have shown that interventional cardiovascular examinations are associated with the highest doses among radiation-related examinations [3-5]. This raises concerns about the radiation risks to both patients and medical staff.
A systematic review on the health effects of occupational radiation exposure conducted in 2017 highlighted that many radiation-related health effects were identified such as cancer, cataracts, circulatory diseases, partial cognitive or olfactory malfunction, carotid atherosclerosis, etc. Among these, cataracts are the most common disease. The incidence of cataract among cardiovascular interventionists is higher than non-interventional cardiologists and radiologists [6]. According to the Society for Cardiovascular Angiography & Interventions (SCAI), up to 50% of interventional cardiologists got changes in their lens, which are precursors to cataracts associated with ionizing radiation [7].
Cataracts are cloudy areas that form in the eye lens, potentially causing blurry vision. They are one of the leading causes of blindness worldwide. Depending on their anatomical locations, cataracts are classified into three types which are (i) nuclear cataracts, which affect the center of the lens. This type of cataract progresses slowly and is primarily age-related; (ii) cortical cataract, which affect the peripheral edge of the lens. Cortical cataracts are slowly progressing and are commonly found in patients with diabetes; (iii) posterior subcapsular cataracts (PSC): cataracts affect the back of the lens. This type of lens opacity progresses faster than the others. PSC was once considered the only radiation-related cataract. However, recent studies found that radiation-induced cataracts may also appear at the peripheral edges of the lens [8,9].
Before 2011, the absorbed dose recommended to cause cataracts was 5 Gy for acute exposure and 8 Gy for protracted exposure. The occupational exposure for eye lens, Heye lens, was 150 mSv/year [10]. However, much epidemiological evidence indicated the increasing incidence of cataracts in people exposed to radiation. Studies conducted on cleaning workers of the Chernobyl accident, survivors after Hiroshima and Nagasaki, patients treated with radiotherapy, and medical staff performing interventional procedures revealed an increased incidence of radiation-induced cataracts, even at doses of the order of 0.5 Gy or no threshold [11,12]. As a result, during a 2011 meeting, the International Commission on Radiological Protection (ICRP) unanimously established a new absorbed dose threshold of 0.5 Gy for cataracts and proposed reducing the occupational dose limit for the eye lens, Heye lens, from 150 mSv/year to 20 mSv/year, averaged over 5 consecutive years, with no single year exceeding 50 mSv [13]. These recommendations have been endorsed by international organizations such as the International Atomic Energy Agency (IAEA), the Health Protection Agency (HPA), and the European Atomic Energy Community (Euratom). Many countries, including Vietnam, have adopted the new recommended dose limits into their radiation safety regulations.
However, the sharp reduction in the dose limit by more than seven times sparked significant controversy, prompting scientists worldwide to conduct more extensive research to estimate exposure levels to the eye lens of medical staff working with radiation and assess the adverse health effects of radiation. A study by Vano et al. in 2010 showed that there is a correlation between radiation dose and cataract incidence. Approximately 38%–53% of cardiovascular interventionists and 21%–45% of medical staff involved in interventional procedures developed PSC [14]. Another study by Jacob et al. also indicated that 17% of surveyed interventionists got PSC [15]. A systematic review conducted by Elmaraezy et al. in 2017 confirmed similar findings, showing that cardiovascular interventional doctors are at a higher risk of developing PSC compared to the control group, with a relative risk of 3.21 [16].
In Vietnam, Circular No. 19/2012/TT-BKHCN, dated November 8th, 2012, issued by the Ministry of Science and Technology, on controlling and ensuring radiation safety in occupational and public exposure, introduced the new occupational dose limit for the eye lens at 20 mSv per year, averaged over five consecutive years with no single year exceeding 50 mSv [17].
In regulation, radiation workers are required to be regularly monitored occupational dose with personal dosimeters. Currently, employee dose monitoring is performed by dosimetry service providers licensed by regulatory agencies. The monitored quantities include dose equivalents such as Hp(10) for estimating effective dose, Hp(0.07) for skin dose, and Hp(3) for eye lens dose. The commonly used personal dosimeter is a passive type (eg. thermoluminescent dosimeter [TLD], or optically stimulated luminescence dosimeter [OSLD]), which is attached to the staff’s body, at chest level. In some cases, it is recommended to wear a second dosimeter at the collar, over the thyroid shielding. The effective dose, skin dose, and eye lens dose are derived from the readings of the personal dosimeters. However, this method may lead to incorrect estimation of the eye lens dose due to the far distance between the dosimeters and the eyes. Moreover, factors such as geometry, energy, and irradiation angles are not considered, leading to large variations in the obtained results. To date, our country has not established instructions on either method or tools for accurate eye lens dose assessment.
In the current context, the reduction of occupational dose limits for eye lens has a significant impact on both medical staff and radiation safety officers in medical centers. Greater attention is required for monitoring and following up on occupational doses, especially Heye lens. This study aims to investigate radiation dose to the eyes of medical staff working in the cardiovascular interventional department, to determine whether the new dose limits are likely to be exceeded, by using OSLDs, and to assess if the personal dosimeters can be used to derive eye lens dose. Recommendations and suggestions are provided to ensure radiation protection for the staff.
2. METHODS
Some methodologies for determining the eye lens dose of radiation workers have been mentioned in a study by Tran et al. [18], including (i) direct measurement by dedicated eye lens dosimeters or dosimeters calibrated to eye lens dose; (ii) indirect estimation through personal dosimeters; and (iii) quick assessment by the correlation between eye lens dose and irradiation duration. To precisely ascertain the eye lens dose, it is essential to determine the average energy of the incident beam and other dependent factors, such as energy distribution, angular dependence, and configuration corrections, etc. This requires a great deal of time and effort to develop methodologies for measuring eye lens dose. Within the scope of this pilot study, the authors employed the first two methods, which are direct measurement with dosimeters calibrated for eye lens dose to directly determine radiation dose for the left and right eyes, and indirect estimation by personal dosimeters to evaluate the average eye lens dose of medical staff. In addition, the correlation between eye lens dose and the irradiation time was also explored.
The study used OSLDs nanoDot type (Fig. 1A) and Inlight type (Fig. 1B) of Landauer manufacturer to monitor radiation dose to eye lens for medical staff. Obtained signals were read with Microstar Reader of Landauer (Fig. 1C). The digital subtraction angiography (DSA) system used in the surveyed procedures was Artis Zee of Siemens manufacturer.

This study was cross-sectional, and the survey reporting conformed to the Reporting of Observational studies in Epidemiology (STROBE) statement [19]. The STROBE checklist was presented in supplemental Table 1.
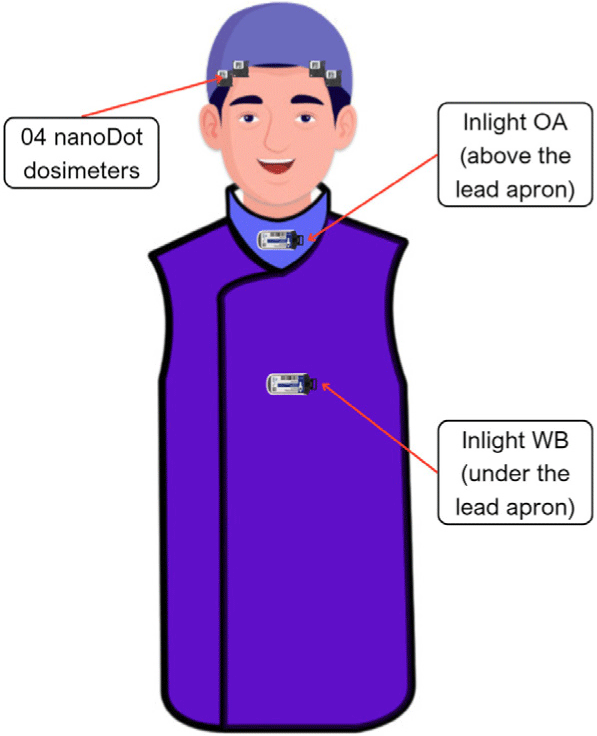
The study was conducted at the Cardiovascular Intervention Department of one hospital in Ho Chi Minh City over a period of 10 weeks (from January 2023 to March 2023). After the content of the study was disseminated, there were 12 interventional staff members consented to take part in the survey including 8 doctors (encoded as A, B, C, D, E, F, G, and H) and 4 technicians (encoded as I, K, L, and M). Dosimeter sets were numbered and labelled for each participant. Before each procedure, participants wore dosimeters at specific positions on their body (Fig. 2), including 4 nanoDot dosimeters attached at the edges on two sides of their surgical caps (2 dosimeters on each side, near the eyes); two Inlight dosimeters, one worn at the chest level, under the lead apron, and another one worn on the collar, over the lead thyroid shield. Dosimeters were read every week with a Microstar Reader, and the readings were allowed to be accumulated for 10 weeks in the dosimeters.
In addition to monitoring the radiation dose to the eyes of staff, the relationship between radiation dose to the eyes and irradiation duration was also investigated by extracting exposure time from a computer at the cath lab.
Eye lens dose equivalent can be calculated by the following formula:
where Hp(3) is the eye lens dose equivalent (mSv); Kair is air kerma (mGy); CFE is the calibration factor at beam energy E; R is the reading of the dosimeter (count); Rbackground is the reading of the background dosimeter (count). Cp(3) is the conversion factor from air kerma to eye lens dose equivalent (mSv/mGy) which can be looked up in Publication 74 of the ICRP 74 [20], or ISO 4037-3:2019 [21]. GCF is the Geometrical Correction Factor, and DRF is the Dose Reduction Factor.
In this study, CFE was taken as 0.0002, the calibration factor for the beam in the diagnostic energy range; R for each eye was averaged from the readings of two nanoDots; Cp(3) was taken as 1.66 (mSv/mGy) – the factor corresponding to the average photon energy of 65 keV, which is the most used energy for X-ray beam in fluoroscopy. DRF was taken as 1 due to no protective equipment being used by the staff, and GCF equals 1 as recommended in the literature [22] resulting in fL equals 1.
The eye dose was calculated by the sum of Hp(0.07) of two Inlight dosimeters. This method was proposed in the recommendations on eye lens dosimetry of the Swiss Society of Radiobiology and Medical Physics [22].
where, Hunder(0.07) và Hover(0.07) are dose equivalents at 0.07 mm depth, taken from the readings of two Inlight dosimeters: WB worn at chest level, under lead apron, and OA worn at the collar, over thyroid shield, respectively (Fig. 2). equals 1 as mentioned above.
Each staff member’s eye lens dose values were illustrated as mean±SD. Mean values were deduced from averaging the reading of 2 dosimeters over at least three reading times. For better analysis and comparison, the participants were categorized into two groups: the doctor group and the technician group. The study analyzed the eye lens dose of doctors and technicians over the 10-week and annual periods and compared them to the prescribed dose limit.
3. RESULTS AND DISCUSSION
Within 10 weeks, a total of 202 procedures were anlayzed, mainly percutaneous coronary intervention (PCI) procedures, radiofrequency ablation, and implantation. Four doctors were responsible as the primary doctors for most of the cases surveyed, including doctors A, B, C, and D. The majority of PCI procedures were assigned to these four doctors. Doctor A is the head of the department and the one who performed the most procedures. Technician M is the head technician of the department.
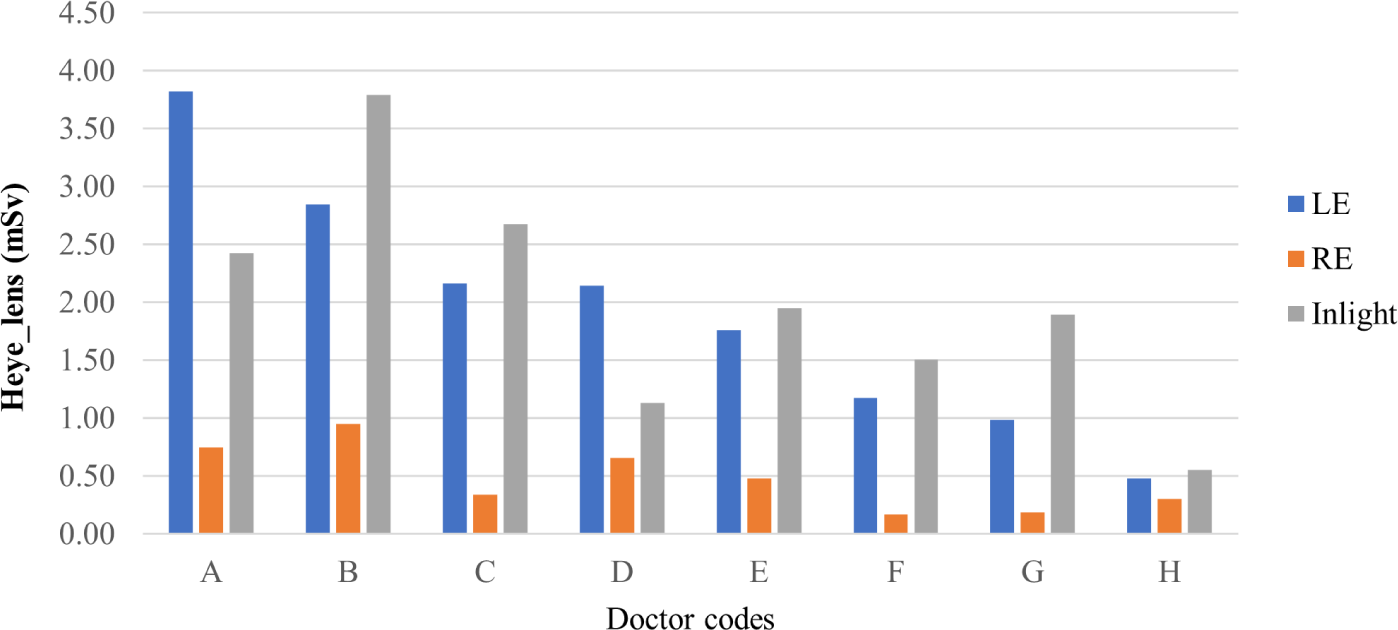
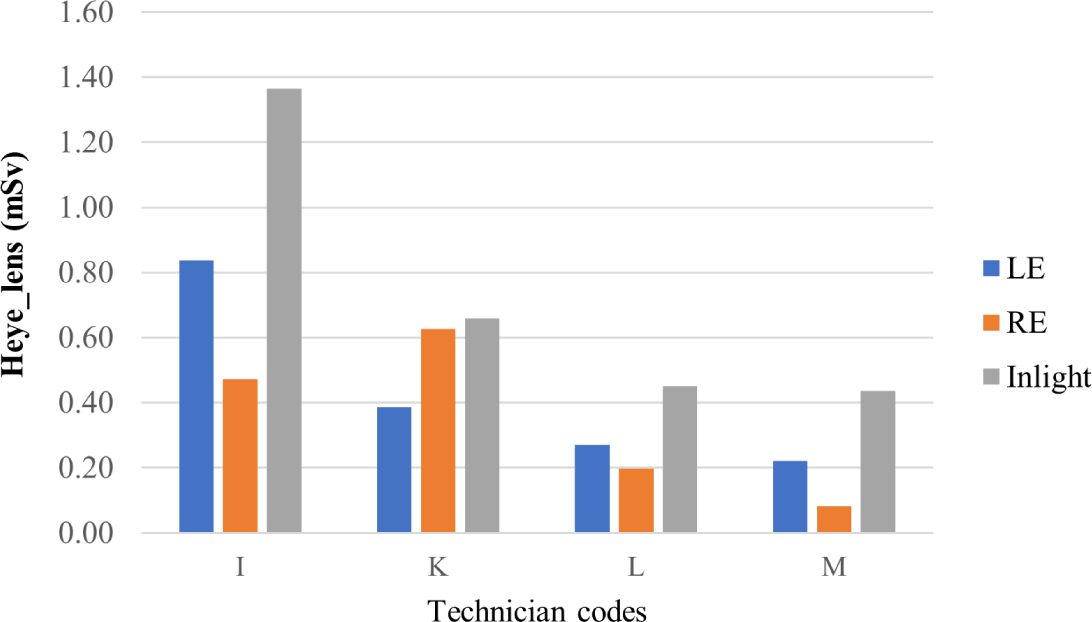
For doctors, the highest and lowest irradiation times were 467 minutes and 160 minutes, respectively. The results for technicians were 1,483 minutes and 194 minutes. Eye lens doses, using the direct measurement method, for the left eye (LE), right eye (RE), as well as the indirect assessment method (Inlight) for both doctors and technicians, are illustrated in Figs. 3 and 4, respectively.
For doctors, the maximum eye lens dose was 3.82±0.71 mSv (left eye of doctor A – the head doctor of the department), and the minimum dose was 0.17±0.03 mSv (right eye of doctor F). The dose to the left eye was significantly greater (p=0.002) than that of the right eye for all the surveyed doctors. This finding is consistent with other studies [23-25]. The reason for this is the standing position of doctors relative to the patient and the X-ray tube, resulting in the left side of the body tending to receive a higher dose than the right side. Not only is the left eye more affected by radiation than the right eye, but another study also demonstrated a greater prevalence of left-sided brain cancer compared to other side in interventional clinicians [26].
For technicians, although their exposure time was nearly double that of doctors, the eye lens doses for technicians were much lower (maximum eye dose of 0.84±0.15 mSv). Additionally, the tendency of doses for the left eye and right eye was not as clear as for the doctors. This is due to technician’s greater distance from the irradiation sources and their frequent changes in standing positions. Sometimes, a technician had to move back and forth between two adjacent DSA rooms. Therefore, determining their eye dose as well as evaluating the relationship between their eye dose and exposure time are complicated tasks. This observation has also been confirmed by similar studies [25-27]. Moreover, for the technician group, unlike doctors, the radiation doses of technicians were not related to their job position. Typically, the head technician had the lowest eye lens dose in the technician group.
Comparing the results of direct and indirect methods, it was observed that for most of the surveyed staff (except doctors A and D), the indirect method with Inlight dosimeters consistently showed higher doses than the direct method, with an average difference of 31%. Exceptionally, for the doctors A and D, the doses from the indirect method were lower than the dose from the direct method. It was explored that Inlight dosimeters were worn at the wrong positions during the first 4 weeks of the survey for these two doctors. From week five, the positions of these dosimeters were adjusted, notwithstanding, the final results were still affected. Therefore, it can be seen that using Inlight dosimeters - the currently used method - may lead to an overestimation of the eye lens dose. In addition, the values obtained from Inlight dosimeters do not specify the dose for the left or right eye. According to other studies, eye lens dose evaluated from personal dosimeter readings gives inaccurate results because the dosimeters are located at a distance from the eyes, and dependent factors such as geometry, energy, and exposure angles are not considered, leading to large errors in the obtained results [28].
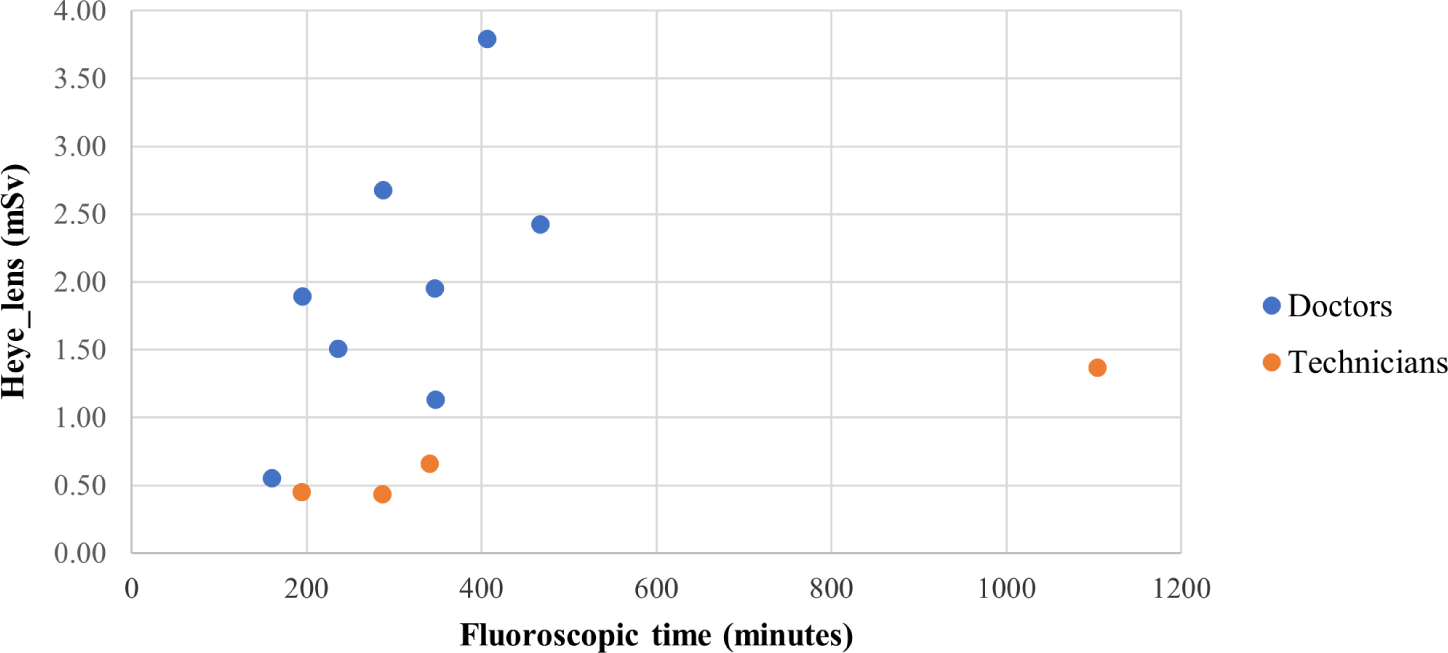
Fig. 5 illustrates the correlation between radiation dose to the eyes of the staff and exposure time. The general trend shows that the longer the irradiation time, the higher the eye lens does. Notably, the sharper increase in eye lens dose relative to exposure time was observed in doctors. The correlation coefficients were 0.62 and 0.99 for doctors and technicians, respectively. A significant correlation was observed in technicians (p=0.01) but not in doctors (p>0.05). This result is likely due to many factors, including the standing position of staff during procedures. Further research on these correlations will be explored in future works.
The annual doses for medical staff were evaluated through the left eye dose to consider the highest risk that the staff may have. It was assumed that there are 50 working weeks in one year. Table 1 illustrates the annual dose for the left eye of staff that was extrapolated from the surveyed dose values (by the direct measurement with OSL nanoDots). For doctors, the annual eye lens doses were from 2.40 mSv to 19.10 mSv. Doctor A received the highest annual dose, which was quite close to the new eye lens dose limit of 20 mSv/year. Meanwhile, the doses for technicians were well below the limit, with the highest annual eye lens dose of 4.20±0.78 mSv. These patterns are consistent with the results of other relevant studies [23-25].
A limitation of this study is the absence of dose reports of emergency cases occurring outside of office hours. Due to resource constraints, the survey was conducted exclusively during office hours. As a result, the survey likely underestimates the actual dose levels received by interventional staff. Therefore, the new dose limit for the eye lens is likely to be exceeded with the current workload of the hospital in this survey.
According to the Guidance on implementation of eye dose monitoring and eye protection of workers, released in 2017 by the International Radiation Protection Association (IRPA), in occupational exposure situations, medical fluoroscopy-guided procedures are likely to be those requiring the most frequent ocular dose monitoring. If the staff has an annual eye lens dose between 6 mSv and 15 mSv, routine eye dose monitoring becomes mandatory, by either indirect or direct method. For staff with an eye lens dose of above 15 mSv/year, more frequent monitoring is required, using appropriate eye dosimeters placed near the most exposed eye [29]. Based on the findings of this study, 5 out 8 doctors (A, B, C, D, and E) had Heye lens higher than 6 mSv, with doctor A received an eye dose of approaching the dose limit. Therefore, these doctors must undergo regular monitoring of their eye lens dose with appropriate eye dosimeters.
In addition, out of 202 surveyed cases, there were only 8 cases in which doctors used suspended shielding. For many reasons, doctors rarely use lead glass in the procedures. Even though there were many studies have show the effectiveness of dose reduction by using personal protective equipment, such as lead glass, for interventional staff [30-32], the frequency of using these items remains very low. This issue is a common reality in most countries in the world, with the given reason being that lead glass interferes and obstructs vision while performing procedures [33,34].
The medical interventional staff, especially those working in the cardiovascular interventional department are highly qualified professionals. They are valuable human resources needed to be maintained and developed in hospitals and medical centers. However, due to the nature of work, some of them may be exposed to high health risk. Therefore, ensuring safety for this workforce is extremely important. It is important to note that using only personal dosimeters to estimate eye lens dose is not enough. It is urgent to implement a routine eye dose monitoring program for staff in units using interventional radiologic equipment, especially interventional cardiology departments, with appropriate dosimeters specified for eyes. To ensure the long-term health of employees, it is necessary to enhance training on radiation safety, improve supervision and monitoring, and encourage the use of protective equipment (lead aprons, protective goggles, lead curtains) during procedures.
4. CONCLUSION
The study surveyed eye lens dose for medical staff working in the cardiovascular interventional department using both indirect and direct methods. The new dose limit for eye lens is very likely to be exceeded with the current workload of the hospital in this survey. In addition, using personal dosimeters – the currently utilized device to monitor occupational exposure - to derive eye lens dose may result in an overestimation.