1. INTRODUCTION
According to statistic from the Vietnam National Institute of Nutrition, 50% of adults living in urban areas have high cholesterol [1]. More alarmingly, the age of onset for these diseases is trending younger due to the increased consumption fast food, fatty foods, and sedentary lifestyle of young people. Dyslipidemia increases the risk of cardiovascular diseases such as atherosclerosis, coronary syndrome, stroke, ....
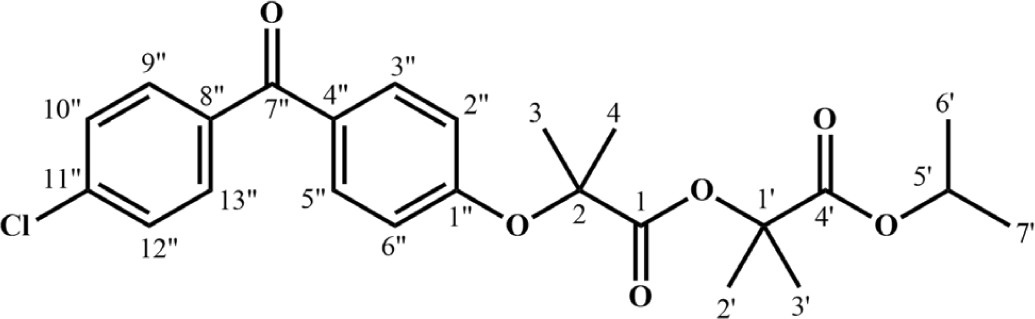
Fenofibrate (Fig. 1) is one of the most used drugs for the treatment of dyslipidemia. In Circular No. 07/2022/TT-BYT dated 05/09/2022 [2], the Ministry of Health stipulates that drugs containing fenofibrate must be evaluated for bioequivalence. According to Circular No. 11/2018/TT-BYT dated 04/05/2018, with regulates the quality of drugs and raw materials [3], the Drug Administration of Vietnam requires that drugs and raw materials must meet the specifications in the reference pharmacopoeia, with standards continuously updated. The Vietnamese Pharmacopoeia, the United States Pharmacopoeia 2022 (USP 2022), and the British Pharmacopoeia 2022 (BP 2022) all require testing for the limits of impurities A, B, and C in fenofibrate raw material [4-6]. The acceptable limits for each impurity are no more than 0.1%, 0.1%, and 0.2%, respectively.
However, neither National Institute of Quality Control nor Institute of Drug Quality Control Ho Chi Minh City provides the fenofibrate impurity C (ImpC) standard. A publication in India reported the synthesis of the ImpC, but the yield and the purity were low [7]. This study employs a response surface methodology (RSM) with a central composite design (CCD) model to optimize the parameters of the synthesis reaction of ImpC and to establish the reference standard of ImpC.
2. MATERIALS AND METHOD
Fenofibrate raw material (batch W-F51-20191102-01, content 100.3% (as dry basis), from Jiangsu Nhwa Pharmaceutical, Xuzhou, China). Isopropyl 2-bromo-2-methylpropanoate (97%), acetonitrile (high-performance liquid chromatography [HPLC] grade) and dimethyl sulfoxide (DMSO) from Fisher Scientific. n-hexane, ethanol, isopropanol, sodium hydroxide, potassium carbonate, hydrochloric acid, phosphoric acid from Merck. All chemicals and solvents were used without further purification.
Mass spectrum was recorded on Ultra Performance Liquid Chromatography (UPLC) system tandem with High Resolution Mass Spectrometry (MS) (Xevo G2-XS QTOF, Waters, Milford, MA, USA). Infrared (IR) spectrum was recorded on IRAffinity-1S (Shimadzu, Kyoto, Japan). The proton nuclear magnetic resonance (1H-NMR) and carbon-13 nuclear magnetic resonance (13C-NMR) spectrum were recorded on Bruker AvanceNEO spectrometer. HPLC was performed on Alliance 2695e system (Waters), equipped with a photodiode array (PDA).
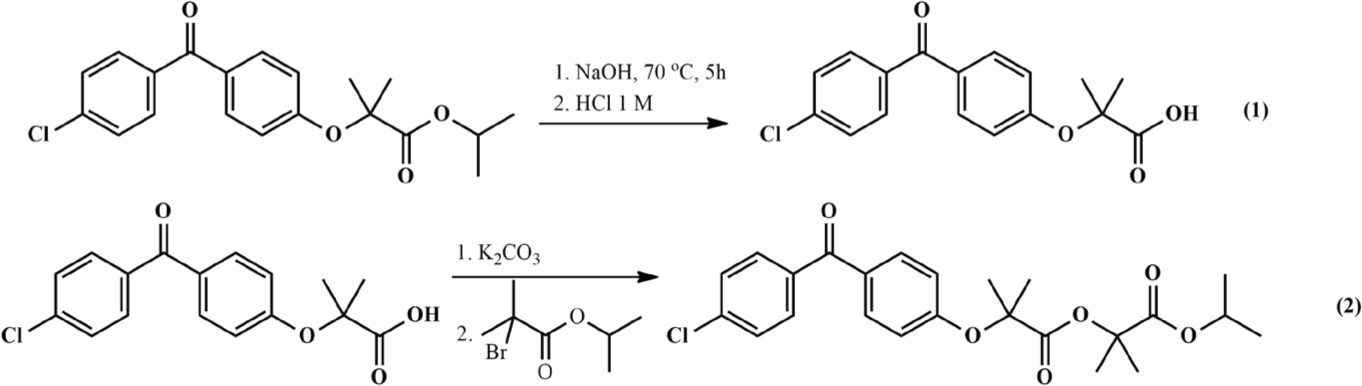
The synthesis process of ImpC was carried out in two steps, starting from the fenofibrate raw material. Fenofibrate was dissolved in ethanol and hydrolyzed with sodium hydroxide for 5 hours at 70℃. Afterward, ethanol was evaporated under reduced pressure and the mixture was acidified by hydrochloric acid. Fenofibric acid precipitated and was filtered. The esterification reaction between fenofibric acid and isopropyl 2-bromo-2-methylpropanoate was performed in DMSO in the presence of potassium carbonate (Fig. 2).
There were four investigated factors of reaction: reaction time (3 to 7 hours), reaction temperature (60℃ to 90℃), mole ratio of potassium carbonate to fenofibric acid (2.0 to 5.0), mole ratio of isopropyl 2-bromo-2-methylpropanoate to fenofibric acid (3.0 to 6.0). In each experiment, 1 mmol of fenofibric acid (318.75 mg) was dissolved in 5 mL of DMSO. Potassium carbonate was added to this solution and the mixture was stirred at 80℃ for 1 hour. Then, isopropyl 2-bromo-2-methylpropanoate was added, and stirring continued. After each experiment, the sample was diluted 100 times in acetonitrile – water (8:2, v/v) and analyzed by liquid chromatography. A Xterra® C18 column (particle size: 5 μm, L×I.D: 150 mm×4.6 mm, Waters) was used for the separation of reaction mixture. All chromatograms were recorded using gradient elution of the mobile phase. The elution started with 51.5% acetonitrile and 48.5% phosphoric acid solution pH 2.5 (v/v) for 12 min and then linear increase the ratio of acetonitrile to 75% for 15 min, after that re-equilibration to initial conditions for 5 min. The signal was acquired at the wavelength of 285 nm. The percentage of the ImpC (%ImpC) peak on chromatogram (by peak area) was recorded.
After reaction, 50 mL of water was added to the mixture and extracted with 50 mL n-hexane. The organic layer was collected, and n-hexane was eliminated under reduced pressure. The crude product (ImpC) was dissolved and purified by recrystallization in isopropanol.
There are two main approaches to process optimization: empirical and statistical methods. The empirical method, also known as the one-factor-at-a-time approach, focuses on changing one factor at a time while holding all others constant [8]. This method has a significant drawback as it ignores interactions between variables. As a result, it does not provide a complete picture of how each parameter affects the outcome. Additionally, this approach requires a large number of experiments, leading to increased time, costs, and resource consumption. Statistical methods, on the other hand, account for these interactions, providing a more accurate picture of how factors work together. They achieve this with fewer experiments, leading to a more efficient and cost-effective optimization process [9].
Several statistical approaches exist for optimizing process variables, including iterative mathematical search, heuristic search, metaheuristic search, simulated annealing, Taguchi, and RSM [10]. RSM is a set of statistical and mathematical techniques used to understand and optimize the relationships between several factors and a response. Its primary objective is to achieve an optimal response. In this research, the CCD model, which is the standard for RSM, was chosen for the optimization of the ImpC synthesis process. The relationship between dependent variables and independent variables can be written as:
where Y is the dependent variable or the response, X1, X2 to Xn are independent variables and ε is the experiment error. Because the independent variables are various in units and/or have different limits of variation, their effects on the response can only be compared after they are coded [11]. In RSM, the relationship between uncoded or actual value and coded form (xi) can be witten as:
where Xi is the actual value of the ith factor in the actual units, X0 is the average of the high and low values of the ith factor, and δX is the step change of X values in the actual units. Temperature (X1), ratio between fenofibric acid and potassium carbonate (Ratio 1–X2), ratio between fenofibric acid and isopropyl 2-bromo-2-methylpropanoate (Ratio 2–X3), time of reaction (X4) were chosen as the independent variables. Their range and levels were given in Table 1.
| Variables | Levels | ||||
|---|---|---|---|---|---|
| −2 | −1 | 0 | +1 | +2 | |
| Temperature (°C) (X1) | 45 | 60 | 75 | 90 | 105 |
| Ratio 1 (X2) | 0.5 | 2 | 3.5 | 5 | 6.5 |
| Ratio 2 (X3) | 1.5 | 3 | 4.5 | 6 | 7.5 |
| Time (hour) (X4) | 1 | 3 | 5 | 7 | 9 |
In this work, four reaction parameters of reaction were evaluated using CCD with the aid of Design Expert Software (ver.11.0.4, Stat Ease, Minneapolis, MN, USA). The analysis of variance (ANOVA) technique was applied to identify significant variables and their interactions on the yield of synthesis reaction. Significant parameters were checked by p-value and F-value; the regression model was checked by using R2 value. Four additional experiments were conducted to verify the validity of the model.
The chemical structure of synthesized product was elucidated from spectral data: IR, MS, 1H-NMR, and 13C-NMR.
ImpC was evaluated by characters: appearance, solubility, melting point, loss on drying, identified by IR, MS, and NMR spectroscopic methods and purity by HPLC.
To evaluate the homogeneity of the bottling process, a random sample of vials was taken, following the formula √N+1, where N is the total number of vials. The percentage of ImpC in each vial was measured using a validated HPLC method. The bottling process was considered homogeneous if the coefficient of variation (CV) for the percentage of ImpC in tested vials did not exceed 0.5%.
Interlaboratory assessment was conducted in 3 independent laboratories that complied with ISO/IEC 17025:2015. Six random samples were sent to each laboratory. The purity of ImpC in random samples was measured. The one-way ANOVA test was performed to evaluate the interlaboratory vial homogeneity. Three chosen laboratories were the Department of Standardization & Reference Materials, the Department of Research and Development, and the Department of Cosmetic Testing of the Institute of Drug Quality Control Ho Chi Minh city.
The assigned value was determined according to the ISO 13528:2022 guidelines [12], based on the results of 18 measurements of ImpC performed by 3 laboratories.
3. RESULTS
A 24 CCD model was generated to investigate the correlation between independent variables and percentage of product in synthesis reaction. In a typical CCD model with 4 variables, the model consists of 16 factorial points, 8 axial points and 6 replicates at the center, 30 runs in total [13]. However, there was one hard-to-change variable in this design (temperature–X1). The software adapted to the desing to include 6 central points, 16 factorial points, 4 hard-to-change axial points and 6 easy-to-change axial points, 32 runs in total. The result of all experiments are listed in Table 2. The reduced-cubic model was selected for the response. The following equation (3) was derived from Design Expert software. This equation shows relationship between four independent variables (X1–X4) and the yield of synthesis reaction (%ImpC):
The ANOVA technique was used to assess the significance of each term in the regression model, the results are shown in Table 3. The significance of each term was determined by the p-value. The smaller the p-value, the more significant is the corresponding term.
The analysis indicated that the model is statistically significant and can explain the relationship between variables and response. All terms in the model have a significant effect to the response, as the p-value is less than 0.05.
Fig. 3 depicts the relationship between actual and predicted value of %ImpC in mixture. The actual values from HPLC data and the predicted values were generated from equation (3). An excellent correlation coefficient (R2=0.9995) was observed between the experimental and predicted responses, indicating that the model fits the results well.
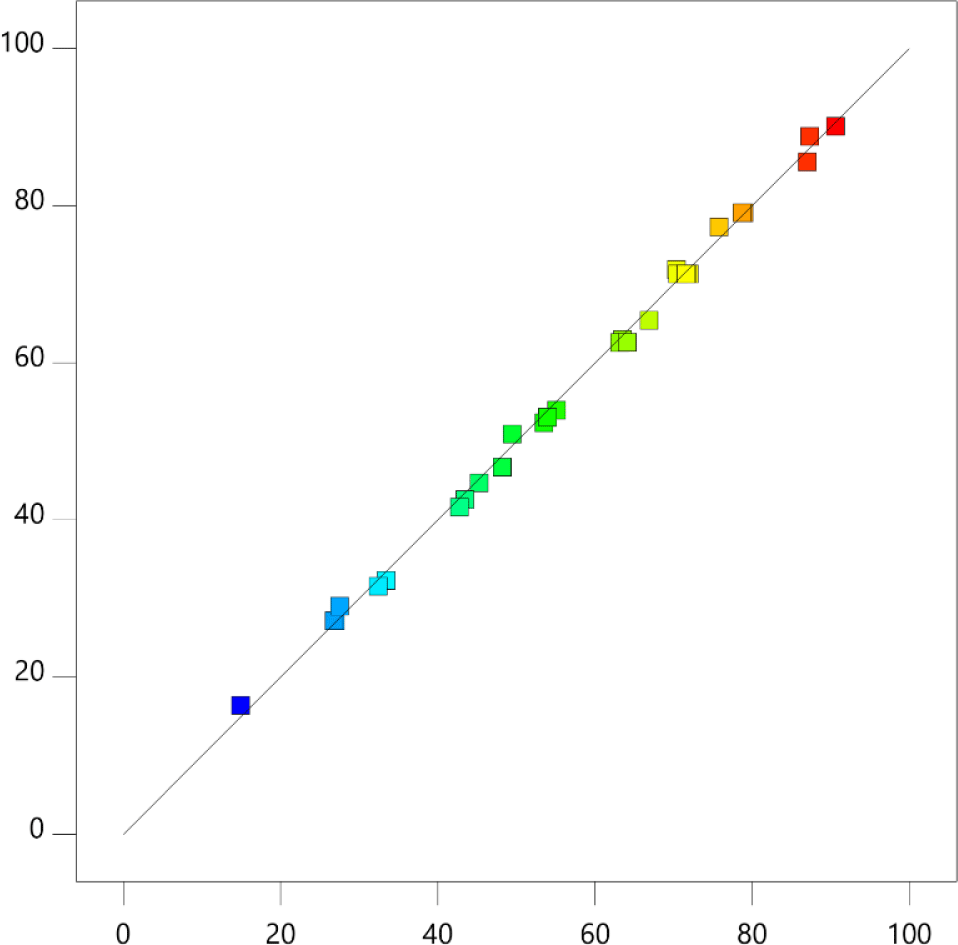
The model was optimized using a reduced-cubic programming approach with the objective of maximizing the percentage of ImpC in mixture after reaction. Four suggested optimization conditions were chosen by software to validate the model. The results of validated experiments are shown in Table 4. All the actual results were close to the predicted value.
| Experiment | Factors | %ImpC | ||||
|---|---|---|---|---|---|---|
| X1 (°C) | X2 | X3 | X4 (h) | Predicted | Actual | |
| 1 | 87 | 4.30 | 5.95 | 3.64 | 90.21 | 89.53 |
| 2 | 87 | 3.86 | 5.60 | 6.78 | 90.04 | 89.30 |
| 3 | 78 | 3.57 | 5.86 | 6.70 | 90.03 | 87.82 |
| 4 | 78 | 4.90 | 4.94 | 6.91 | 90.33 | 86.99 |
Mass spectrum of ImpC measured in the positive-ion mode showed a major [M+H]+ peak at m/z=447.15778, this value corresponds to the accurate monoisotopic mass of 446.1496, indicating a molecular formula of C24H27ClO6.
IR spectrum displayed characteristic absorption peaks of functional groups, including: 2,978 (C-Hsp3), 1,736 (C=O ester), 1,659 (C=O, conjugated ketone), 840 (C–Cl).
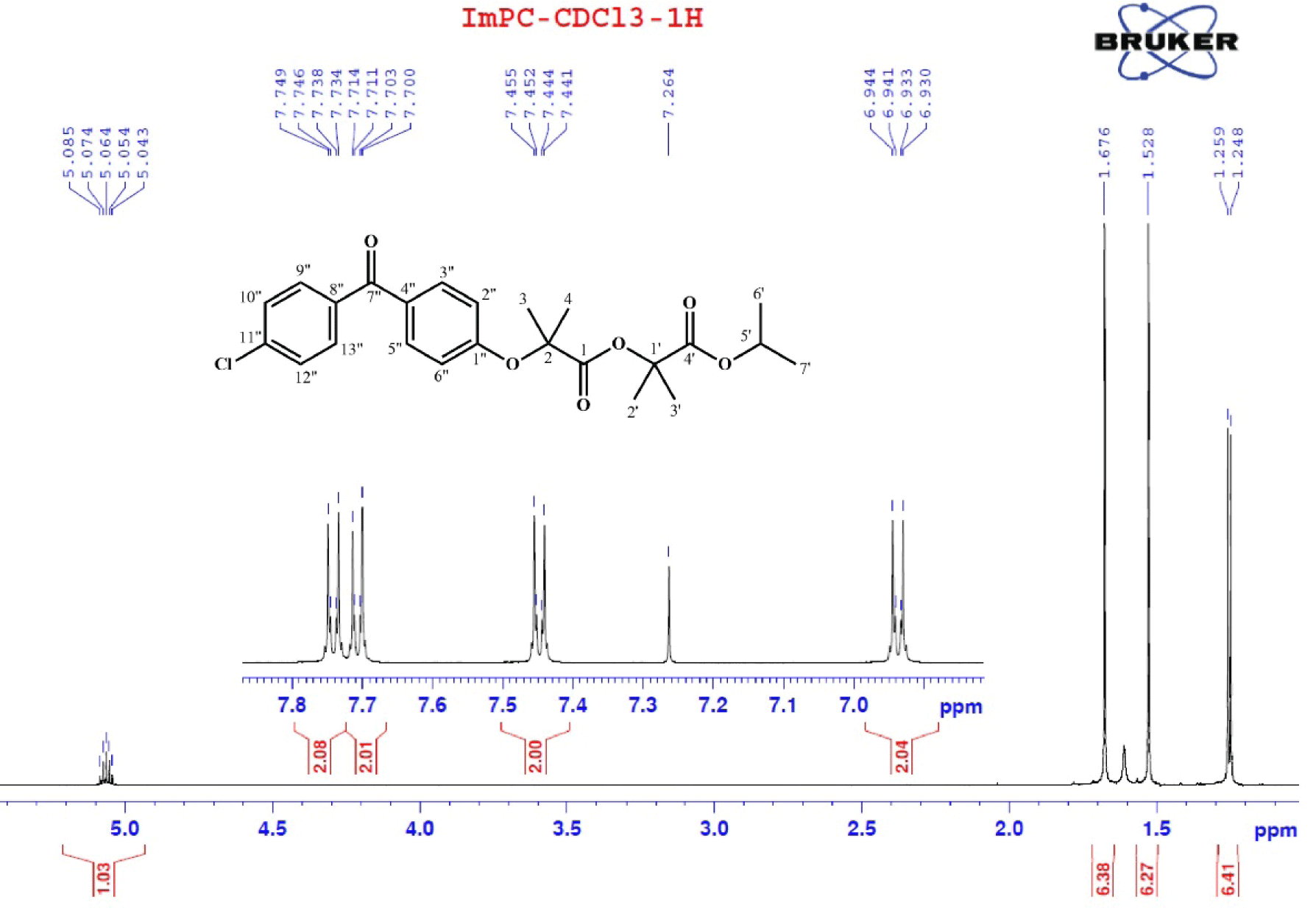
1H-NMR spectrum had total integration number of 27, indicating that synthesized compound has 27 hydrogen atoms. Signals on 1H-NMR were (600 MHz in CDCl3, δ ppm, Fig. 4): 7.75–7.73 (m, 2H, H9”, H13”); 7.71–7.70 (m, 2H, H3”, H5”); 7.46–7.44 (m, 2H, H10”, H12”), 6.95–6.93 (m, 2H, H2”, H6”), 5.07 (sept, J=6.6 Hz, 1H, H5’), 1.68 (s, 6H, H3, H4), 1.53 (s, 6H, H2’, H3’), 1.25 (d, J=6.6 Hz, 6H, H6’, H7’).
Data on 13C-NMR (150 MHz in CDCl3, δ ppm, Fig. 5): 194.3, 172.2, 171.4, 159.5, 138.4, 136.5, 131.9, 131.2, 130.5, 128.6, 117.8, 79.9, 79.6, 69.1, 25.4, 24.27, 21.62.
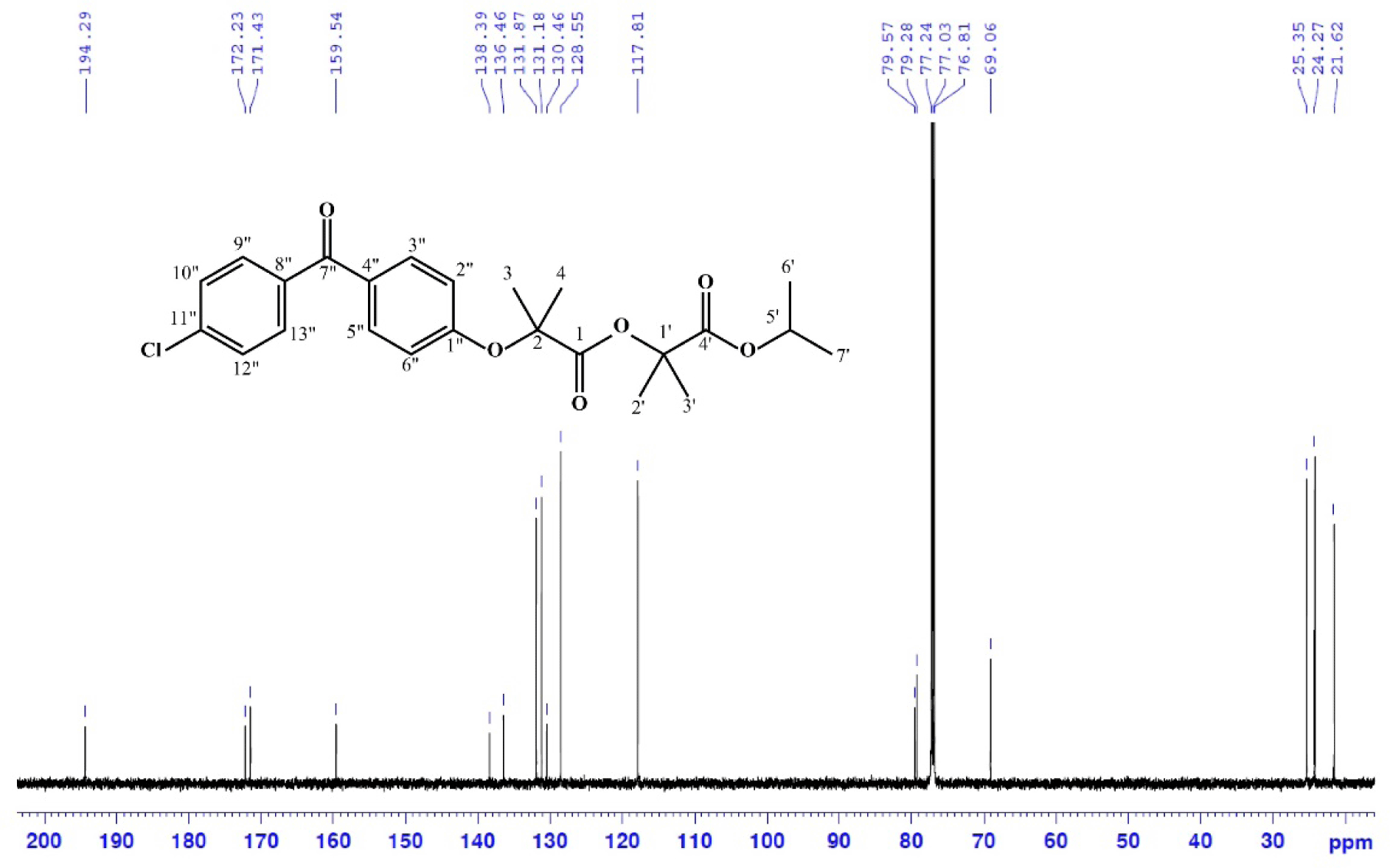
Table 5 shows the assessment results of ImpC. As the chromatographic purity was above 99%, the synthesized ImpC meets the requirements for establishing a reference substance.
| Character | Method | Criteria |
|---|---|---|
| Appearance | White crystal, odorless | |
| Solubility | Insoluble in water, sparingly soluble in methanol, freely soluble in ethyl acetate | |
| Melting point | DSC | 106.5°C |
| Loss on drying | TGA (up to 250°C) | 0.15% |
| Identity | IR | ν (cm–1): 2,978.1 (C-H alkane); 1,735.9 (C=O ester); 1,658.8 (C=O conjugated ketone); 1,242.1 (C-O ester) |
| MS | HRMS (+): m/z=447.15905 [M+H]+; m/z=469.14521 [M+Na]+; m/z=273.06878 [M-C8H13O4]+ | |
| NMR | NMR data were suitable with Figs. 4 and 5 | |
| Purity | HPLC | >99.0% on the basis |
Nine hundred milligrams of ImpC were packed into 90 vials, with 10 mg each. Ten vials were chosen randomly (using Excel software) to evaluate the vial homogeneity. Another 18 vials were also collected randomly (using Excel software) to evaluate interlaboratory vial homogeneity. The results of vial homogeneity and interlaboratory vial homogeneity are shown in Tables 6 and 7, respectively.
| Vial | Purity of ImpC (%) |
|---|---|
| 15 | 99.478 |
| 02 | 99.483 |
| 23 | 99.486 |
| 39 | 99.481 |
| 48 | 99.473 |
| 51 | 99.486 |
| 69 | 99.484 |
| 21 | 99.485 |
| 66 | 99.475 |
| 04 | 99.486 |
| Average | 99.482 |
| CV (%) | 0.005 |
The CV% of the 10 vial was 0.005%, indicating that the bottling process met the requirements for homogeneity. The difference between the purity of ImpC obtained from the threelaboratories was not statistically significant (p=0.60) (Table 7). Therefore, analytical procedure was highly reproducible and the purity of ImpC was independent of the participating laboratory.
The result of determining the assigned value of ImpC was shown in Table 8. After three iterations, there was no change in the s* value (s*=0.009). The assigned value of ImpC was 99.483%.
4. DISCUSSION
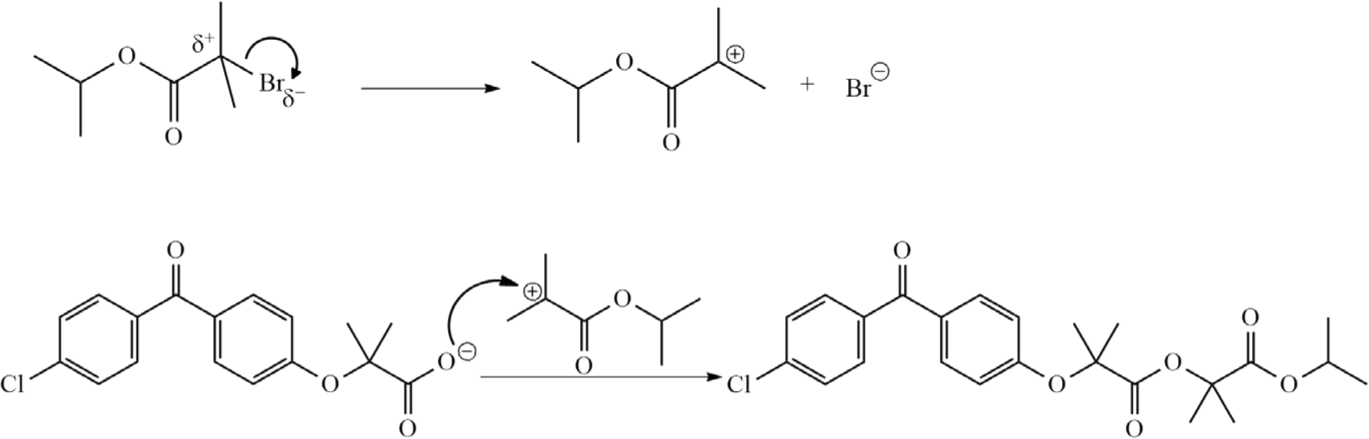
The conversion of fenofibric acid to ImpC was a two-step process. First, fenofibric acid reacted with potassium carbonate to form potassium fenofibrate. The next step was the SN1 reaction between the carboxylate ion and isopropyl 2-bromo-2-methylpropanoate to generate ImpC (Fig. 6).
The halide derivative used in the esterification reaction is a tertiary derivative. So, the substitution reaction occurs via the SN1 mechanism [14]. The SN1 reaction proceeds with the slow step being the formation of the carbocation, which determines the rate of the reaction. The second step is the attack of the carboxylate ion on the carbocation formed in the first step. The formation of the potassium salt plays an essential role in the esterification reaction. Because carboxylic acid is a much weaker nucleophile than the carboxylate, if fenofibric acid is not converted to the carboxylate form, the rate of the reaction to form an ester will be reduced significantly. In an experiment lacking presence of potassium carbonate, ImpC was not produced.
The type of base used in the first step was also investigated. Using potassium hydroxide resulted in a lower yield than potassium carbonate (both were used in a 1:1 molar ratio with fenofibric acid). This result can be explained by the water formed from the reaction of potassium hydroxide with fenofibric acid, which inhibits the esterification reaction. The water produced can also create an environment for the hydrolysis of ImpC, or it can hydrolyze the halide derivative, isopropyl 2-bromo-2-methylpropanoate. Potassium carbonate is a two-step base, when reacting with fenofibric acid at mole ratio higher than 1, the reaction only proceeds through the first step. Thus, it does not produce water that affects the next reaction. In addition, the strong alkalinity of potassium hydroxide can hydrolyze the raw materials and ImpC to form other byproducts.
The objective of this study was to optimize the conditions for the synthesis of ImpC. To build a robust and highly reproducible model, the dependent variable needed to be carefully selected and accurately recorded. The selected dependent variable was the product composition (%ImpC) in the post-reaction mixture, which was determined using the HPLC method. The HPLC analysis method has high specificity, which is clearly shown in the chromatogram of the post-reaction mixture (Fig. 7). All the components in the mixture are completely separated from each other. This method also has high accuracy, so the results obtained from the experiments have high repeatability (as shown in Table 2, %ImpC in six replicated experiments being quite close).
RSM is characterized as a statistical technique that utilizes quantitative data gathered from suitable experiments to address multivariate equations. A key benefit of RSM is its ability to extract a wealth of information from a relatively small set of experiments. By building mathematical models and generating visual representations, RSM allows us to analyze the individual and combined effects of different variables on the desired outcome. This powerful technique not only identifies the factor levels that lead to the optimal response, but it can also manage scenarios with multiple responses, ultimately determining the best overall conditions [9,13]. There are various techniques in RSM including CCD, Box–Behnken design (BBD), and Full Factorial Design (FFD) [11]. This study investigated four reaction factors, each with three levels of values (Table 1). The number of experiments required for the FFD, CCD, and BBD were 81, 32, and 30, respectively. We did not choose the FFD because it required too many experiments. The CCD and BBD models had a similar number of experiments. Although CCD requires two more experiments than BBD, many studies have shown that the CCD model provides more accurate results than BBD [15-17]. Therefore, the CCD model was chosen to optimize the synthesis process of ImpC. A reduced cubic model was developed to predict the reaction yield as a function of the individual and interactive parameters. There was an excellent regression between independent and dependent variables (R2=0.9995).
The result in Table 3 shows that all the individual variables have a significant impact on the model as the p-value below 0.0001. In general, higher of the value of variables, the higher yield of ImpC was generated. The reaction factors do not act independently, but also interact with each other on the reaction yield.
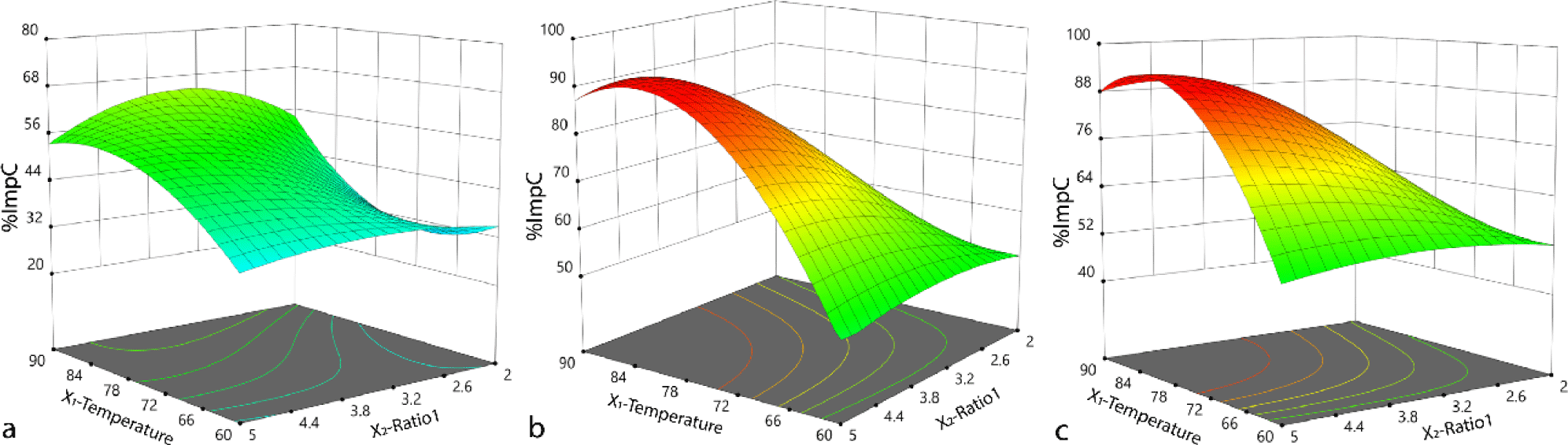
As we mentioned earlier, the formation of ImpC follows the SN1 mechanism, and therefore the reaction rate is determined by the concentration of the isopropyl 2-bromo-2-methylpropanoate in the reaction mixture. The effect of ratio of acid fenofibric and isopropyl 2-bromo-2-methylpropanoate (X3 or Ratio 2) at fixed reaction time of 5 hours was shown in Fig. 8a and b. At low X3 value, the response surface plot does not show any conditions for %ImpC higher than 70%. Conversely, when X3 is increased to higher values, the maximum of %ImpC displayed on the response surface plot can reach up to 95%. From the result in Table 3, the reaction time (X4) also has a significant impact on the yield reaction. The longer the reaction time, the higher the amount of ImpC generated. However, as the concentration of isopropyl 2-bromo-2-methylpropanoate increases, the reaction rate also increases, so it was not necessary to extend the reaction time to reach the high yield. At high X3 values, a reaction time of 4 hours can already achieve the yield of up to 90% (Fig. 8c). These observations are consistent with the optimal conditions selected from Table 4.
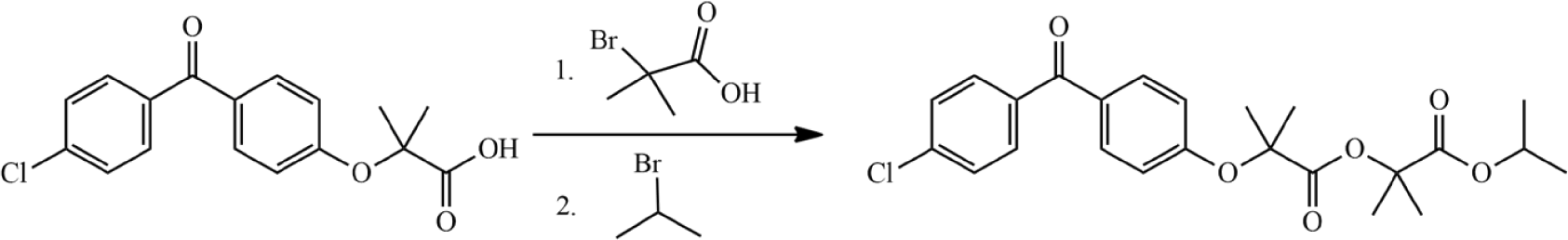
In a previously published study, Patil et al. [7] synthesized ImpC from fenofibric acid via two esterification reactions with the agents 2-bromo-2-methylpropionic acid and isopropyl bromide, respectively (Fig. 9). The authors performed the reaction in acetonitrile at room temperature, with a total reaction time of up to 24 hours and the yield of the synthesis process was 12%. In this study, we directly synthesized ImpC via the reaction between fenofibric acid and isopropyl 2-bromo-2-methylpropanoate in the presence of potassium carbonate (Fig. 2(2)). The selection of a suitable reaction agent eliminated an unnecessary intermediate step, thereby contributing to reducing the reaction time and increasing the reaction yield. Furthermore, the optimization process with the assistance of Design Expert software helped establish the correlation between reaction factors and yield of reaction. The suggested conditions from the software were validated through practical experiments. The results indicated no significant difference between theoretical and experimental outcomes. The yield of ImpC was approximately 90%, with a reaction time of less than 4 hours (Table 4, Experiment 1).
1H-NMR spectral data of ImpC showed similar signals to those published in the literature [7], but its 13C-NMR spectral data has not been published yet. Compared to the structure of fenofibrate, the structure of ImpC has one more carbon of ester, one quaternary carbon, and two methyl groups. All these differences were revealed in the 13C-NMR spectrum of ImpC when compared to the 13C-NMR data of fenofibrate [18]. There was an additional signal in the region 170.0–173.0 ppm (ester); one more signal which has δC around 79.0–80.0 ppm (R3C-O) and one more signal at 21.0–25.0 ppm (2 symmetrical methyl groups). Therefore, it can be concluded that the synthesized product was ImpC.
5. CONCLUSION
The study identified a regression model linked the conditions and the yield of the reaction to synthesize ImpC from fenofibric acid and isopropyl 2-bromo-2-methylpropanoate. The optimal conditions were selected with a reaction time of less than 4 hours and a reaction yield of approximately 90%. ImpC was standardized as a reference substance. The assigned value of the reference substance was 99.483% and uncertainty measurement (u) was 0.02%. Compared to a previous study [7], the reaction yield has been significantly improved. In addition, the use of an optimization model helped identify the optimal conditions with a significantly reduced reaction time.
The synthesis and standardization of ImpC contributes to enriching the source of national standard substances. This helps manufacturers and administrators access a reiable source of standard substances to strictly control the raw material of fenofibrate used in production. These efforts directly contributes to improving the quality of drugs and ensuring safety for patients.