1. INTRODUCTION
Methotrexate (MTX) is a chemotherapy and immunosuppressive agent widely used to treat cancer, autoimmune diseases in children and adult patients, and ectopic pregnancy [1-3]. However, MTX is highly toxic to the liver, kidney, and nervous system [4,5]. People receiving long-term MTX treatment should be monitored for side effects, especially for those with insufficient kidney function [6-9]. Pharmacokinetic monitoring of the MTX in patients under MTX therapy may optimize the treatment efficacy and reduce the adverse effects of MTX [10]. Determination of MTX levels in plasma contributes to adjust MTX dosing.
MTX concentrations are usually quantified in the laboratory using an automated immunoassay analyzer. However, metabolites of MTX and other endogenous compounds can induce cross-reactivity in immunoassays. Previous studies showed that all MTX concentrations were affected by 7-hydroxy methotrexate (a metabolite of MTX in plasma), as quantified by immunoassays such as FPIA (fluorescence polarization immunoassays) and EMIT (enzyme-multiplied immunoassay technique) whereas HPLC method was not affected [11,12]. Various methods for the quantification of MTX and its metabolites in plasma by HPLC, liquid chromatography-mass spectrometry (LC-MS), capillary electrophoresis, etc. have been developed and validated [13]. HPLC is considered the standard method for the accurate quantification of MTX concentrations in plasma collected from cancer patients treated with high doses of MTX [14].
Although MTX is widely indicated in cancer treatment as well as ectopic pregnancy in Vietnam, MTX levels have not been monitored during treatment. Based on the actual need to monitor the therapeutic toxicity of MTX in order to optimize the therapeutic effect and control the toxicity of the drug, the purpose of this study is to quantify the concentration of MTX in human plasma using HPLC connected to PDA detector.
2. MATERIALS AND METHOD
The SPE cartridges (OASIS® HLB cartridge 1 mL were obtained from Waters (USA). Superspher® 100 RP-18 (C18 reversed-phase HPLC column) was purchased from Merck (Germany). Liquid chromatography was performed on HPLC system 2695 XE (Waters) equipped with a PDA detector.
Para aminoacetophenone (PAAP) and MTX were from Sigma Aldrich. HPLC-grade methanol (MeOH) was purchased from Merck and JT Baker, respectively. Potassium dihydrogen phosphate, ammonium hydroxide solution 25% and formic acid were obtained from Merck. Human plasma samples for analytical development were obtained from Blood Bank of Cho Ray Hospital at Ho Chi Minh City, Vietnam. The Ethics Committee of University of Medicine and Pharmacy at Ho Chi Minh City has already approved this study.
Reagents: Solutions of 4% phosphoric acid and 5% methanol were prepared by diluting the concentrated solutions with double distilled water. Phosphate buffer (pH 6.0) was prepared by diluting 0.68 g KH2PO4 in double distilled water to obtain 500 ml of 0.01 M KH2PO4. pH (6.0) was adjusted with 0.2M KOH.
Stock solutions: The solutions of 1 mg/mL PAAP (as internal standard) and 100 μg/mL MTX prepared separately in 0.01 M NaOH were considered as stock solutions, and then stored at -20 °C. Working solutions of 10 μg/mL PAAP and MTX were diluted from the stock solutions with 0.01 M NaOH.
Calibrations and quality control samples: MTX calibrations were prepared by adding 100 μL of 10 μg/mL PAAP as into 400 μL of plasma fortified appropriate amounts of MTX to achieve calibrators concentrations of 0.5, 1, 5, 15, 25 μg/mL. MTX quality control (QC) samples consisted of 100 μL of PAAP 10 μg/mL and 400 μL of plasma fortified appropriate amounts of MTX to achieve QC concentrations of 0.5, 10, 20 μg/mL.
These samples were then processed similarly to the real samples. For samples obtained from patients under MTX therapy, only 100 μL of 10 μg/mL PAAP were added to 400 μL of plasma.
100 μL of 10 μg/mL PAAP, 400 μL of 4% H3PO4 and 500 μL of plasma were added to a 2 ml eppendorf tube. Plasma samples were mixed for 30 min and then centrifuged at 10,000 xg for 10 minutes at room temperature to remove protein precipitates. 800 μL of supernatants were transferred into OASIS HLB cartridge 1ml previously activated with 1 ml of pure MeOH and 1 ml of distilled water. Next, the impurities were removed from cartridges using subsequently either 1 ml of distilled water or 5% MeOH and 1 ml of 2% HCOOH. Then MTX and internal standard (IS) simultaneous extraction was performed by adding either 1mL of pure MeOH or 5% ammonium hydroxide in MeOH. The solution eluted from the cartridge was collected into a 1.5 mL eppendorf and evaporated to dryness at 50 °C. The dry fraction was reconstituted with 200 μL of 0.01M NaOH. After centrifuging at 10,000 xg for 5 min at room temperature, 10 μL of the supernatants were collected for MTX measurement in HPLC system.
Chromatographic separation of the extracted samples was obtained using C18 reversed-phase column Superspher® maintained at 40 °C. The tray temperature in the autosampler was kept at RT. The isocratic mobile phase contained 80% of 0.01M KH2PO4 (pH 6.0) and 20% of pure MeOH (v/v). The flow rate was set at 1 mL/min. The time of isocratic separation was 10 min after injecting 10 μL of sample onto the column and then a gradient mode was used for 5 min to clean the column before the next run.
The validation criteria were according to the U.S. Food and Drug Administration (FDA) guidelines 200 [15].
Selectivity: MTX and IS were spiked in blank plasma samples to differentiate the analytes from other components in the matrix. The responses of interfering components should not exceed 15% of QC levels of MTX and 5% for IS.
Linearity: The linearity of the method was assessed on the 5 calibrators in a replicate of 3 in 3 runs. The coefficient R2 of the linear curve should be > 0.99. Calibration curves were based on the ratios of MTX/PAAP peak area versus MTX concentrations. The regression equation was used to calculate plasma MTX levels. The coefficient of variation (CV) should not exceed ± 20% at lower limit of quantification (LLOQ) and within ± 15% at the other concentrations.
Accuracy: The accuracy was assessed with QC samples at 3 levels of 0.5, 10, 20 μg/mL in replicate of five. Intra and inter-assay accuracies were calculated from the differences between the nominal and the observed concentrations. The within-run CV% value of accuracy should be ≤ 15% at QC levels and ≤ 20 % at LLOQ.
Precision: The precision was assessed with QC samples at 3 levels of 0.5, 10, 20 μg/mL in replicate of five. The within-run CV% value of precision should be ≤ 15% at QC levels and ≤ 20 % at the LLOQ.
Recovery: Recovery of MTX was determined by comparing the results from extracts at 3 QC levels (0.5, 10, 20 μg/mL) with MTX standard concentrations in replicate of five.
LLOQ and LOD (limit of detection): LLOQ is a concentration with CV% of precision and accuracy ≤ 20%, and ratio of signal/noise (S/N) > 10:1. LOD is a concentration with S/N > 3:1.
Stability: The stability of MTX was investigated in triplicate in QC samples at 0.5, 10, 20 μg/mL, after 12h at RT. The stability of MTX and IS in the autosampler was evaluated after 24h at 4 °C. MTX and IS were stable in plasma if the change in concentration did not exceed ± 15% from the original concentration.
Statistics were performed using Microsoft Excel 2010. The results were expressed as mean ± SD (standard deviation). The significance of the slope and intercept of the linear regression equation T-test was evaluated by T-test [16]. The overall significance of the regression analysis was evaluated by F-test [17]. The difference was significant with P value < 0.05.
3. RESULTS
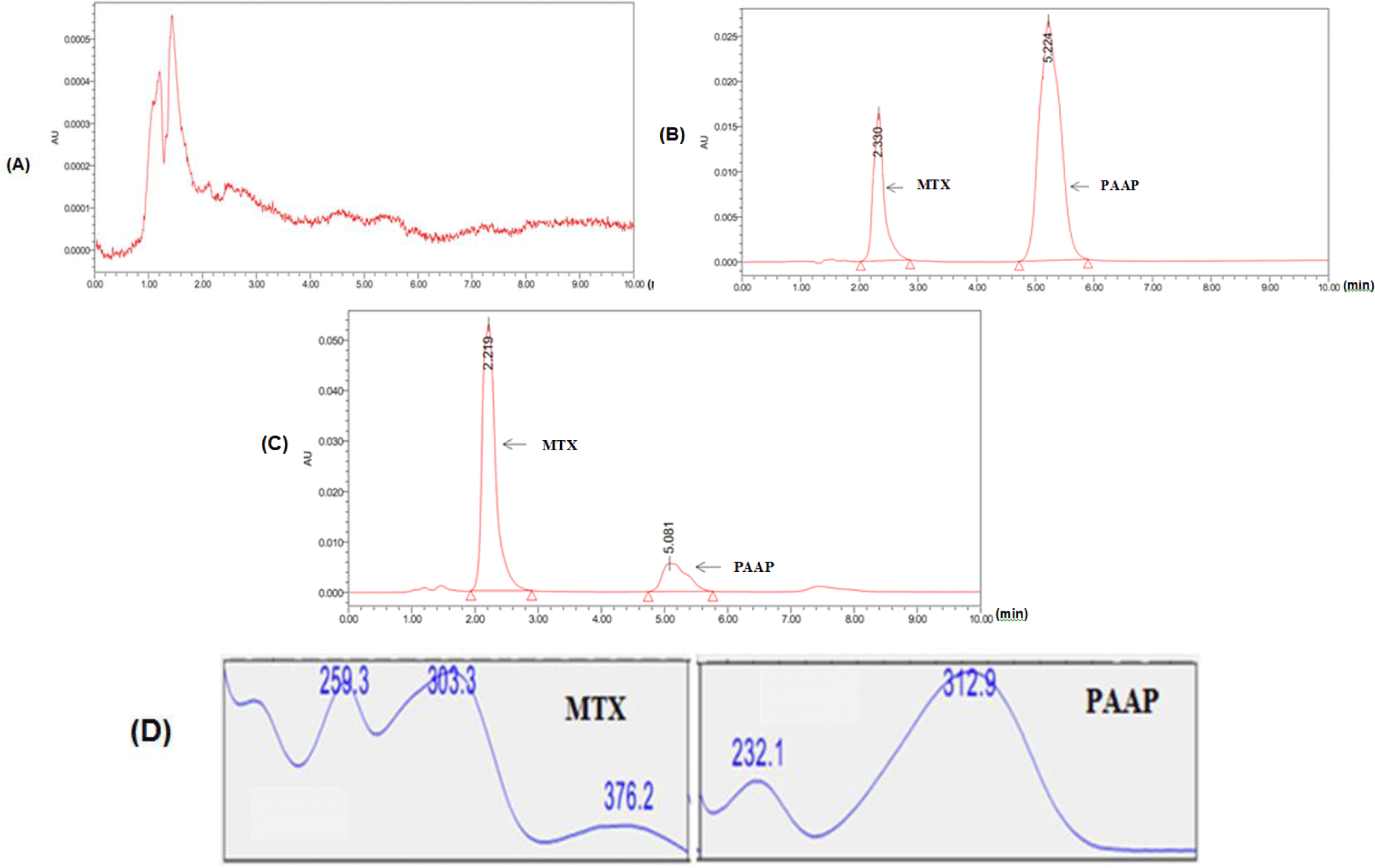
After transferring the plasma samples onto SPE cartridge, 1 ml of 5% methanol and 1 ml of 2% HCOOH were subsequently added to the SPE. Then SPE was eluted once with 1 mL of pure MeOH. MTX signals were detected with a high intensity by HPLC system. This suggested that the washing and eluting steps were sufficient to remove impurities from MTX in plasma. Based on HPLC conditions, diode array HPLC detector (PDA) showed the maximum absorbance of MTX at 303 nm.
Selectivity: MTX and IS were observed at 2.3 min and 5.2 min, respectively, in chromatogram. No interfering peak was detected at retention times of interests. Retention times and spectra of MTX and IS in standard mixture and QC samples were the same (see Figure 1).
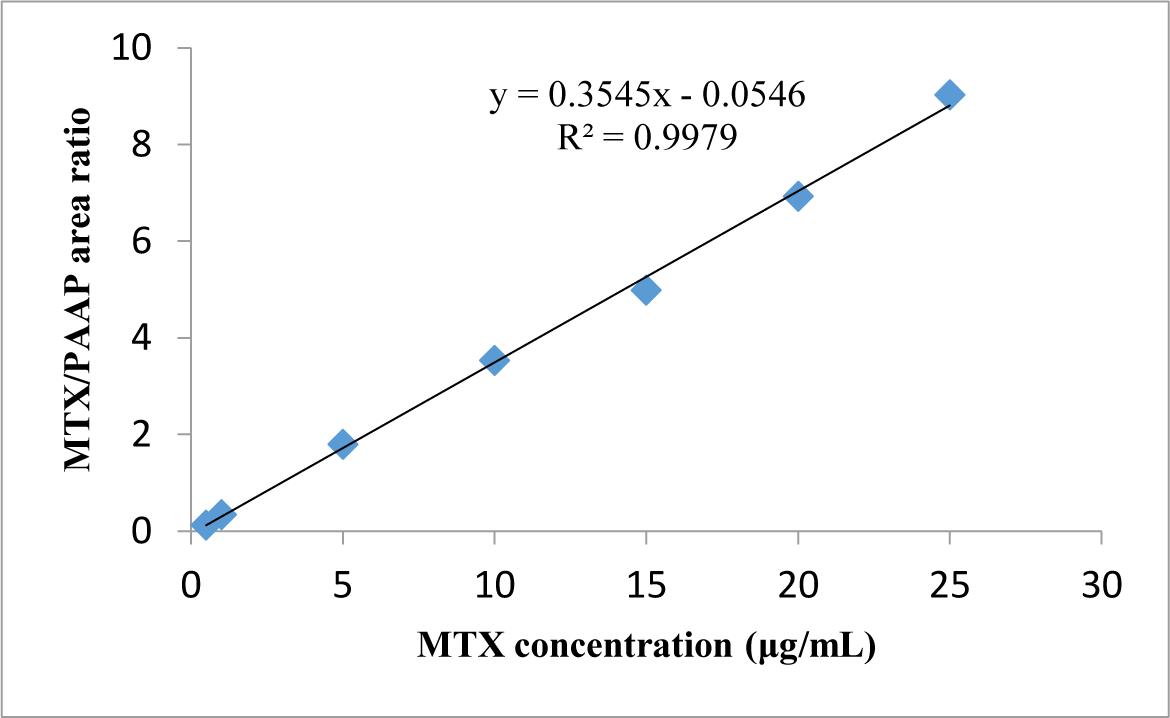
Linearity: The linear concentration range of MTX was from 0.5 to 25 μg/mL. The typical linear regression equation between MTX concentration (μg/mL) and ratio of MTX/PAAP peak area was: y = 0.3545x - 0.0546 with the mean value of coefficient of determination (R2) for MTX calibration curve > 0.997 (see Figure 2).
Accuracy and Precision: At the 3 QC concentrations mentioned above, CV% of the between-day and within-day precision ranged from 2.93 – 8.1% and from 4.5 – 7.44%, respectively. The between-day and within-day accuracies were in the range from 100.04 – 106.65% and 101.05 – 105.02%, respectively. All inter-and intra-day assays are summarized in Table 1.
Recovery: Average recoveries at three QC concentrations of MTX above 77% (range: 70.08–81.98%) displayed a good reproducibility (see Table 1).
Limit tests: LLOQ of MTX was found to be 0.5 μg/mL with accuracies in range from 94.79 – 103.68% and imprecision at 3.63%. LOD concentration was 0.075 μg/mL, respectively (see Table 2).
Stability: MTX levels in the initial plasma samples were insignificantly different from plasma samples after 12h at RT, and extracts maintained the autosampler for 24h.
4. DISCUSSION
MTX is used in various dosages in the treatment of different diseases. Therefore, the risks of MTX toxicity depend on dose and duration of administration. It was recommended that plasma concentrations of MTX should be controlled to ≤ 1.0 μM (equivalent to μg/mL) after initiation of a high-dose MTX for 42 hours. Plasma concentrations of 5 μg/mL MTX at 42 hours after initiation of a high-dose MTX infusion could lead to MTX toxicity [18,19]. The previous studies demonstrated that MTX and its insoluble metabolites could induce acute nephrotoxicity [6,7].
Because a relationship between the outcome of MTX treatment and its systemic exposure has been demonstrated, monitoring of MTX concentrations in plasma may prevent early occurrence of adverse reactions and to monitor the optimization of patient clinical response [20]. To date, many quantitative methods have been developed to measure MTX plasma concentrations such as HPLC) equipped with either ultraviolet (UV) or PDA (Photodiode array) or mass-spectrometric (MS) detectors, or immunoassays such as ELISA (enzyme-linked immunosorbent assay), FPIA and EMIT [21-24]. The method most commonly used for MTX measurement is FPIA. However, the plasma MTX concentrations as quantified by immunoassays were affected by 7-hydroxy methotrexate (a metabolite of MTX in plasma) whereas HPLC method was not affected [11,12]. The choice of the method may depend on different factors such as the equipment available in the laboratory, the experience of the laboratory technicians and the number of assays to run. In previous studies, many HPLC methods using C18 reversed-phase columns were developed to determine MTX concentrations in plasma [2,19,21,23-25].
Although MTX is widely indicated in cancer treatment as well as ectopic pregnancy in Vietnam, MTX levels have not been monitored in treatment. Based on the actual need to monitor the therapeutic toxicity of MTX in order to optimize the therapeutic effect and control the toxicity of the drug, we developed and validated a reliable HPLC method to quantify MTX concentrations in plasma. LLOQ of 5 μg/mL was similar to that of previous chromatographic methods [19,26] and highly reliable as it was determined based on the signal/noise ratio and met the validation requirements. We have developed an HPLC method with low sample injection volume and short analysis time (retention times of 2.3 min for MTX and 5.2 min for PAAP) compared to previous methods [19,29]. The limitation of this study is that the HPLC method developed to quantify MTX concentrations has not yet been used for bioequivalence studies and clinical trials. Therefore, this HPLC method can be used for further bioequivalence studies of MTX or to measure the MTX concentration in plasma from patients under MTX therapy.
Conclusion
We described a simple and reliable HPLC method suitable for quantifying plasma MTX concentrations with LLOQ 0.5 μg/mL. This method met the required validation criteria including: selectivity, linearity, accuracy, precision, recovery and stability, thus, may be used for further bioequivalence studies of MTX or to measure the MTX concentration in plasma from patients under MTX therapy.
