1. INTRODUCTION
Hydnophytum formicarum Jack. is a species of Hydnophytum (Rubiaceae), growing on Phu Quoc Island, South of Vietnam. The tubers of H. formicarum have traditionally been used as a decoction or an aqueous extract for the treatment of rheumatism, arthritis, liver and intestinal diseases [1]. Our previous study has shown that extracts of H. formicarum possessed in vitro antioxidant and cytotoxic activities [2]. The 50% ethanolic extract of H. formicarum (EEHF) has been proved no acute toxicity in Swiss albino mice at the maximum oral dose (Dmax) of 10 g/kg [3]. This extract expressed the in vivo anti-inflammatory and analgesic effects as well as hepatoprotective activity at the oral doses of 100 mg/kg and 200 mg/kg in mice [3-5]. However, there is no evidence for the potential toxicity of this extract in long-term use. Therefore, the present study aims to investigate the subacute toxicity of EEHF in order to provide the confidence of its safety for human use.
2. MATERIALS AND METHOD
The tubers of H. formicarum were collected from Phu Quoc forest, Kien Giang province, Vietnam in February 2014 and authenticated by Prof. Assoc. Thi Dep Truong, Department of Botany, Faculty of Pharmacy, University of Medicine and Pharmacy at Ho Chi Minh City, Viet Nam. The tubers were dried, ground to powder and extracted with 50% ethanol under percolation protocol, then concentrated to dryness (the average humidity of EEHF is 7.33%).
Healthy Swiss albino mice of both sexes with age of 6 - 7 weeks, weighing 28 - 32 g from the Nha Trang Institute of Vaccine and Medical Biology, IVAC (Nha Trang, Vietnam) were used. The animals were fed on free access to food pellets (IVAC, Nha Trang, Vietnam) and water ad libitum, maintained at room temperature (27 ± 3 °C), with a 12h light/dark cycle. The mice were acclimatized to laboratory conditions for 1 week before the onset of the experiment. All experimental protocols were carried out under agreement of the scientific committee, specialty of Pharmacology and Clinical Pharmacy, University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam (Decision No. 1138/QÐ-ÐHYD on the 9th May 2016).
The study on subacute toxicity of the EEHF was conducted for 30 and 60 days according to the guideline for preclinical and clinical trials of traditional medicines and natural products of Vietnamese Ministry of Health [6]. For this study, 46 healthy adult mice of both sexes (50% males and 50% females) were used and randomly divided into 3 groups. Groups I (n = 14) served as control group and received distiller water, the vehicle to dissolve the EEHF. Groups II and III (n = 16) were daily administered with the EEHF at the doses of 100 and 200 mg/kg body weight per day, respectively, for 60 consecutive days using oral gavage. The clinical observation was carried out daily for 60 days and the body weight of tested mice was measured weekly. The animals were measured for the final body weight and then anaesthetized, sacrificed on the 30th day for 8 mice per group and on the 60th day for the rest of the test animals. The blood samples from cardiac puncture and the organs (liver, kidney) were collected for biochemical, hematological and histological analysis.
The blood samples were placed in two groups of test tubes. A half of the test tubes containing anticoagulant, ethylene diaminotetraacetic acid (EDTA) determined the hematological parameters (red and white blood cells, hemoglobin, hematocrit, MCV, MCH, MCHC, platelets, lymphocytes, monocytes, eosinophils, neutrophils and basophils). The other half without anticoagulant centrifuged on the electrical centrifuge to obtain sera for the tests of renal and hepatic functions (ALT, AST, urea, and creatinine). The blood samples were analyzed at the Medlatec General Hospital - Ho Chi Minh City clinic.
After gross pathological observation, the organs were fixed in 10% buffered formalin, underwent several liquor baths of increasing degree (80%, 95% and 100% ethanol), before passing into xylene and being included in the liquid paraffin. The paraffin blocks were mounted on a microtome to make the cuts. Histological sections (5 μm thicknesses) were soaked in aqueous dyes (Hematoxylin and Eosin) and observed under light microscope.
The data were presented as mean ± S.E.M (standard error of the means) and statistically analyzed by Kruskall-Wallis and Mann-Whitney tests, using SPSS version 22 with 95% confidence interval. The difference between groups was considered statistically significant at p value < 0.05.
3. RESULTS
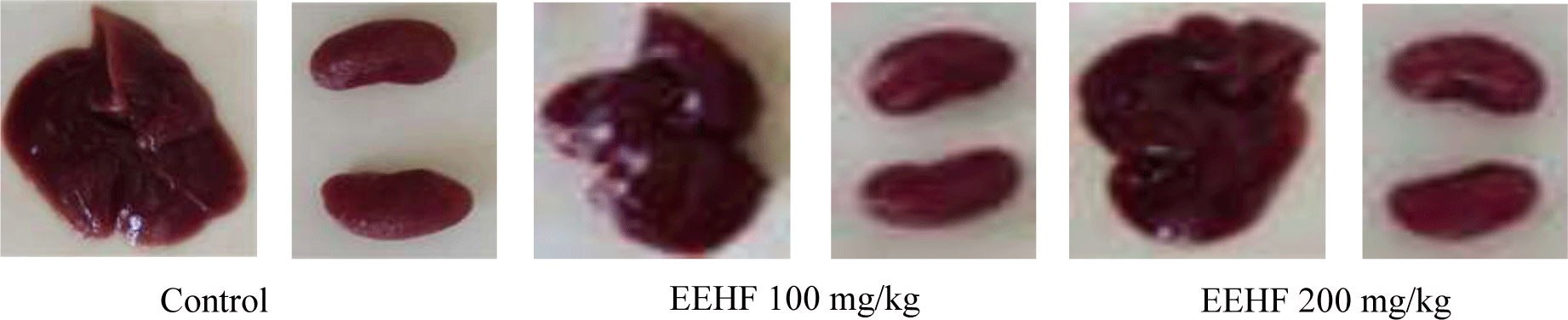
Throughout the study period, no sign of toxicity and mortality was observed on EEHF-treated mice with the oral doses of 100 mg/kg and 200 mg/kg for 60 consecutive days. The gross observation of the livers and kidneys of the treated animals showed no significant changes compared with the control group (Figure 1). There was no abnormality in the liver color or texture in EEHF-treated mice compared to the controls. The livers of all animals were red, smooth, neither edema nor congestive. The kidneys of three groups were red, normal, without expression of any congested or damaged signal.
The oral administration of EEHF at tested two doses (100 and 200 mg/kg) during 60 days caused no significant change on the body weight of mice compared to controls (p > 0.05, Table 1).
The effects of EEHF on red blood cell parameters are presented in Table 2.
After 30 and 60 days of the oral administration at the doses of 100 mg/kg and 200 mg/kg, no significant difference on red blood cell parameters was observed between the treated group and the control, except increases in RBC and Hb after 30 days and decreases in Hb and MCH after 60 days of the group treated with 200 mg/kg EEHF compared to control (p < 0.05). However, the red blood cell parameters of two EEHF-treated groups were within the reference range for mice [7].
The effects of EEHF on white blood cell parameters are presented in Table 3. There was no significant change in white blood cell parameters in the EEHF-treated groups (100 mg/kg and 200 mg/kg) compared to the control, after 30 and 60 consecutive days of treatment.
The effects of EEHF on platelet parameters are shown in Table 4. The EEHF did not express any effect on platelet parameters after 30 and 60 consecutive days of the oral administration.
The effects of EEHF on biochemical parameters are presented in Table 5. In the subacute toxicity study, the liver function parameters of the mice received 100 mg EEHF/kg for 30 or 60 days and 200 mg EEHF/kg for 30 days were within the reference range for mice [7] and were not significantly different from the control group (p > 0,05). The oral gavage of EEHF at the dose of 200 mg/kg for 60 consecutive days could increase AST and ALT levels in comparison with the control (3.2 and 3.9 fold, respectively, p < 0.05). In detail, about 50% mice treated with 200 mg EEHF/kg had ALT and AST parameters within the reference range for normal mice [7].
The oral administration of EEHF at two doses of 100 mg/kg and 200 mg/kg for 30 or 60 consecutive days indicated a non-significant change on kidney function parameters like urea and creatinine levels which suggested that the EEHF did not cause toxicity for the kidneys.
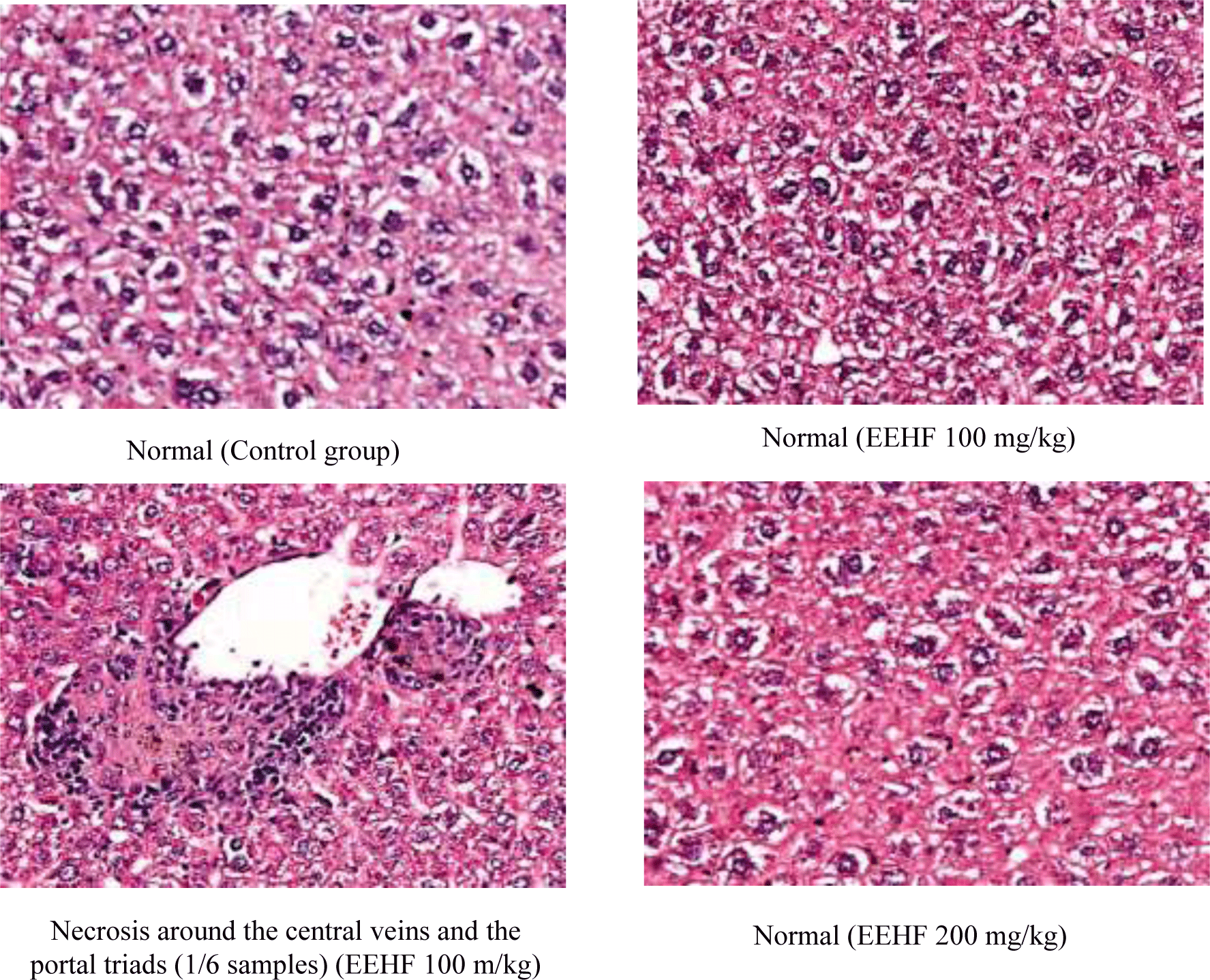
The histological aspects showed normal hepatic tissue after oral administration of 100 mg/kg or 200 mg/kg EEHF and water in the experimental duration of 60 days. Only 1/6 sample of the 100 mg/kg EEHF-treated group show minimal hepatitis with necrosis around of portal triads and central veins (Table 6 and Figure 2).
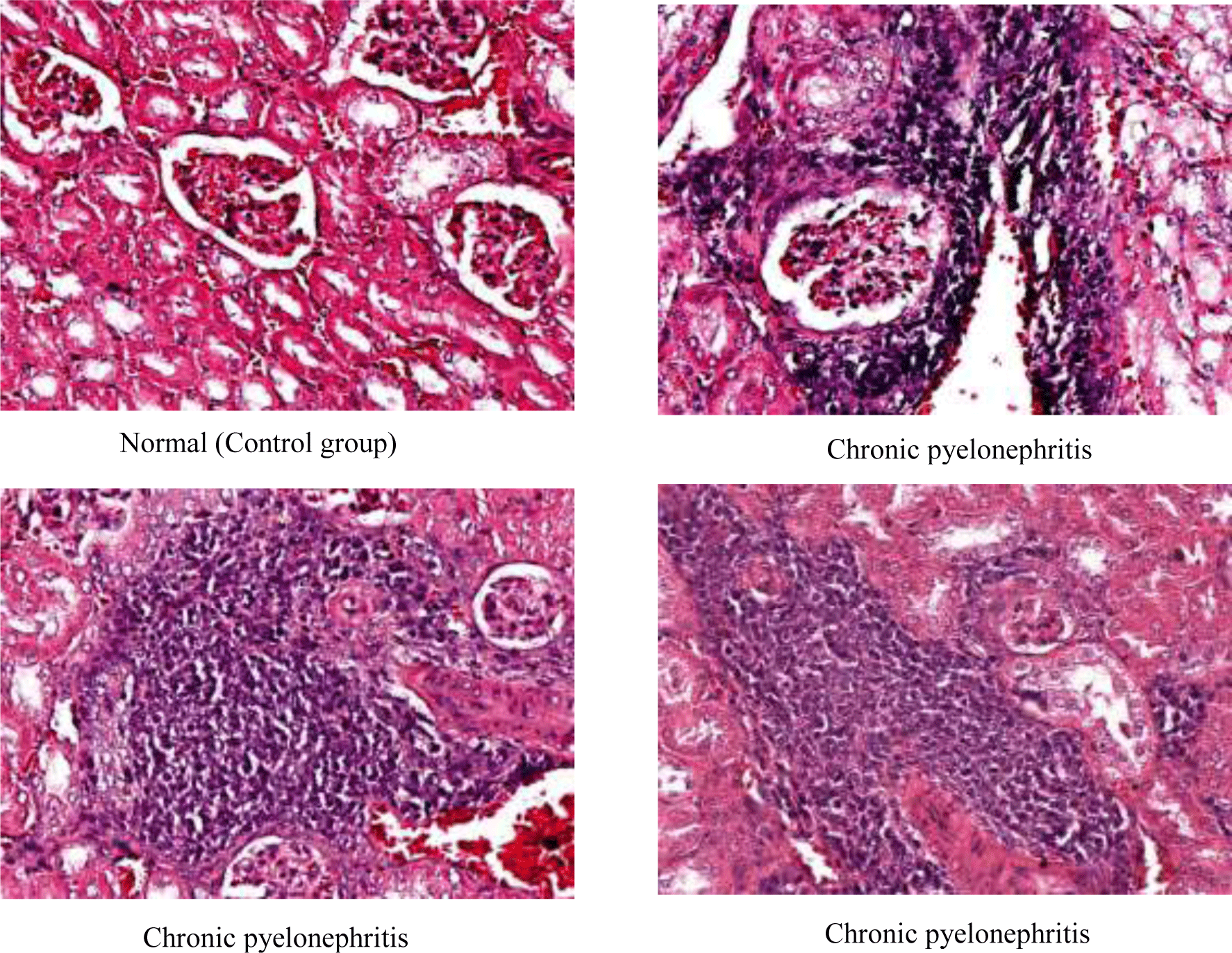
After 60 days of the experiment, histopathological studies of the kidney’s sections of mice treated with EEHF at the doses of 100 mg/kg and 200 mg/kg showed chronic pyelonephritis similar to the controls. Almost of mice kidney sections revealed chronic pyelonephritis (Table 6 and Figure 3).
4. DISCUSSION
Plants’ curative effects have been either recorded in books or simply passed from generation to generation by word of mouth, gradually forming people’s usage habit. According to World Health Organization, 80% of the people living in rural areas depend on medicinal herbs as primary healthcare system [8]. These plant products are in continuous demand and have an edge over other products in the area of drug discovery and therapeutics due to their high efficacy, safety and easy availability [8]. However, there is lack of evidence and awareness of potential toxicity of the plant-derived medicines. The acute and sub-acute toxicity studies are needed not only to determine the appropriate doses for pharmacological studies but also to provide the evidence of the safety of natural products in the clinical use.
Our previous studies reported that EEHF expressed in vivo anti-inflammatory and analgesic effects as well as hepatoprotective activity at the oral doses of 100 mg/kg and 200 mg/kg in mice [3, 4, 5]. The oral dose of 100 mg EEHF/kg could be chosen to extrapolate the therapeutic dose in the human. In this study, the subacute toxicity was evaluated at the daily dose of 100 mg/kg corresponding to estimated dose in the human and 200 mg/kg (two folds of estimated therapeutic dose) to examine possible toxic signs. According to the guideline for preclinical and clinical trials of traditional medicines and natural products of Vietnamese Ministry of Health, the test of subacute toxicity should extend a period of 4 folds of therapeutic time [6]. The EEHF has shown the hepatoprotective effect after 2 weeks treatment. Hence, this subacute toxicity study examined accumulated toxicity caused by repeated oral dosing over 60 days in mice. The mice treated with EEHF at the dose of 100 mg/kg showed no signs of morbidity and mortality. During the experiment, no death and no apparent behavioral changes were observed in both treated groups compared with the control group.
The change in body weights could serve as an indicator of general health status of mice. During 60 days of the experiment, the EEHF-treated mice showed gradual normal increase in their body weight. There was no significant difference in mean body weight of all groups. This result can reveal the positive health status of the animals [9].
The hematological parameters could be one of the sensitive and important markers of toxic substances [10]. In this study, no important changes were observed in red blood cells, white blood cells and platelets indicators in mice treated with the EEHF at the dose of 100 mg/kg. Similarly, all biochemical parameters of hepatic and renal functions did not show significant changes. This corresponded to gross pathological and histopathological examinations of the liver and kidneys. There was no significant difference in color, shape, size, texture and microscopic structure of treated mice compared to controls. The results of the subacute toxicity study suggest that the EEHF at the dose of 100 mg/kg is safe and could be used for therapeutic purposes.
5. CONCLUSION
The results of the subacute toxicity study of the 50% ethanolic extract of the H. formicarum tubers did not affect adversely the body weight, hematological and biochemical parameters at the oral dose of 100 mg/kg. There was no adverse effect in the liver and kidney of treated mice. These results provide the evidence of the safety of H. formicarum tubers to develop health products from this medicinal plant for pharmaceutical uses.