1. INTRODUCTION
Actinomycosis is an uncommon and chronic progressive infection caused by the Actinomyces genus, which are commensal inhabitants of the oropharynx, gastrointestinal tract, and female genital tract. Actinomyces spp. acquires its pathogenicity through tissue trauma, for example following a surgery or an insertion of an intrauterine device (IUD) [4]. Only about 92 reported cases of IUD-related pelvic actinomycosis exist in the published English language literature, although there are 300 million patients a year using IUD [11]. Ileocecal, appendix, and ovary are the most involved regions in this form of actinomycosis [8, 11]. As far as we know, there are only very few case reports on actinomycosis involving psoas muscle and all of them are unilateral [11, 13].
We hereby report a rare case of left psoas abscess caused by Actinomyces spp., which later migrated to the right side in a patient who just has her IUD removed.
2. CASE PRESENTATION
A 35-year-old woman was admitted to our hospital with abdominal pain and difficulty in stretching her right leg due to two masses in the bilateral iliac fossae.
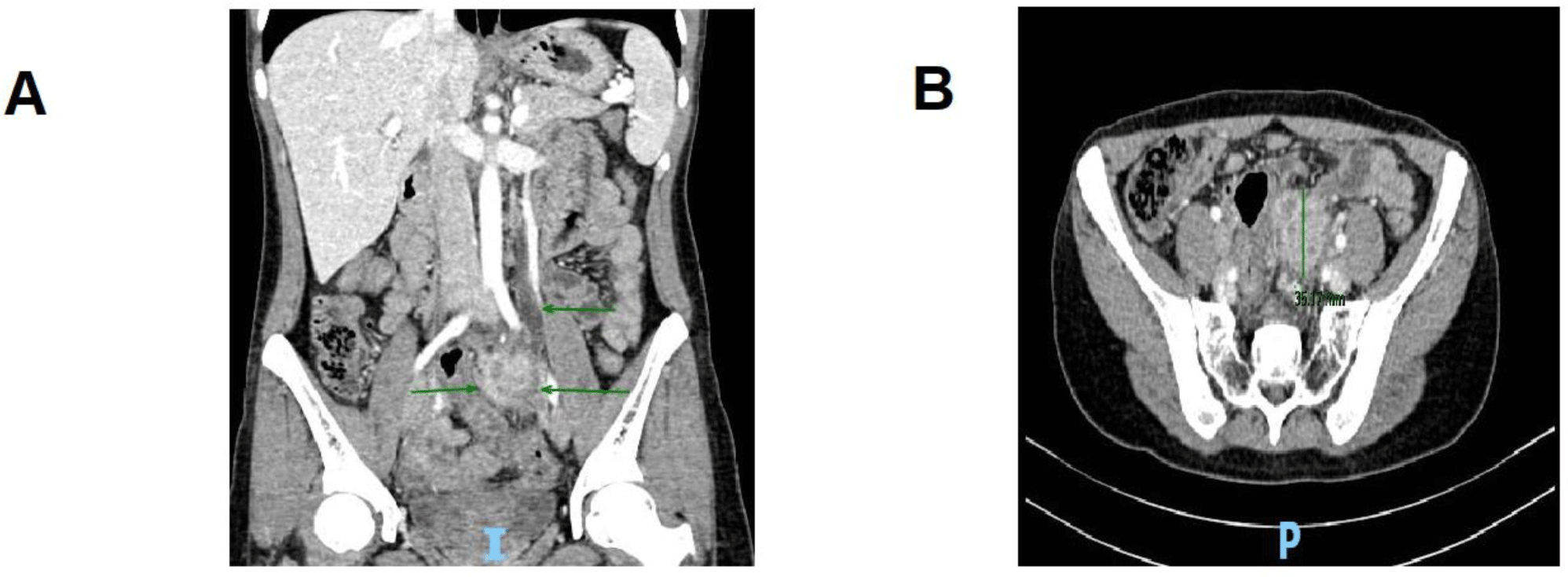
Five months prior to this admission, she went to a gynecology outpatient clinic to annually check her IUD. Because of its wrong position, her IUD was removed but in a difficult manner that required four attempts. Four months before this admission, she developed mild abdominal pain located in the umbilical region without any fever, nausea, or diarrhea. Her symptoms were not alleviated with painless over-the-counter medications, thus she presented to our emergency department, University Medical Center, Ho Chi Minh City. The abdominal computed tomography (CT) scan revealed a 3 x 4 cm2 mass located just below the left common iliac artery with the internal gangrene (Figure. 1). It also caused a left ureteral obstruction which resulted in mild hydronephrosis. The examination only showed mild tenderness on deep palpation of the left iliac fossa. Laboratory data demonstrated a raised C-reactive protein (CRP) (31.1 mg/L) and mild anemia (Hb 98 g/L). Because of her personal problem, she suggested that we transfer her to Cho Ray hospital. During the first admission at Cho Ray hospital, an exploratory laparotomy was performed due to the high suspicion for malignancy. The histopathology result of this mass was inconclusive, and she was discharged.
Two months prior to this admission, she came back to Cho Ray hospital due to persistent abdominal pain and difficulty in stretching her left leg. Imaging studies were similar to the first admission except for the severe left hydronephrosis. She underwent the second exploratory laparotomy to take a biopsy of this mass again and insert a double-J stent into the left ureter at the same time. The biopsy result was undetermined again with only chronic inflammatory cells, and no signs of malignancy. After that, her pain was not only unrelieved but also migrated to her right iliac fossa, causing fixed flexion deformity of her ipsilateral leg. One and a half months after her second surgery, she was admitted to our hospital again.
She had no personal or family history of remarkable diseases. Seven years ago, she was inserted the IUD as a contraceptive method after her second vaginal delivery.
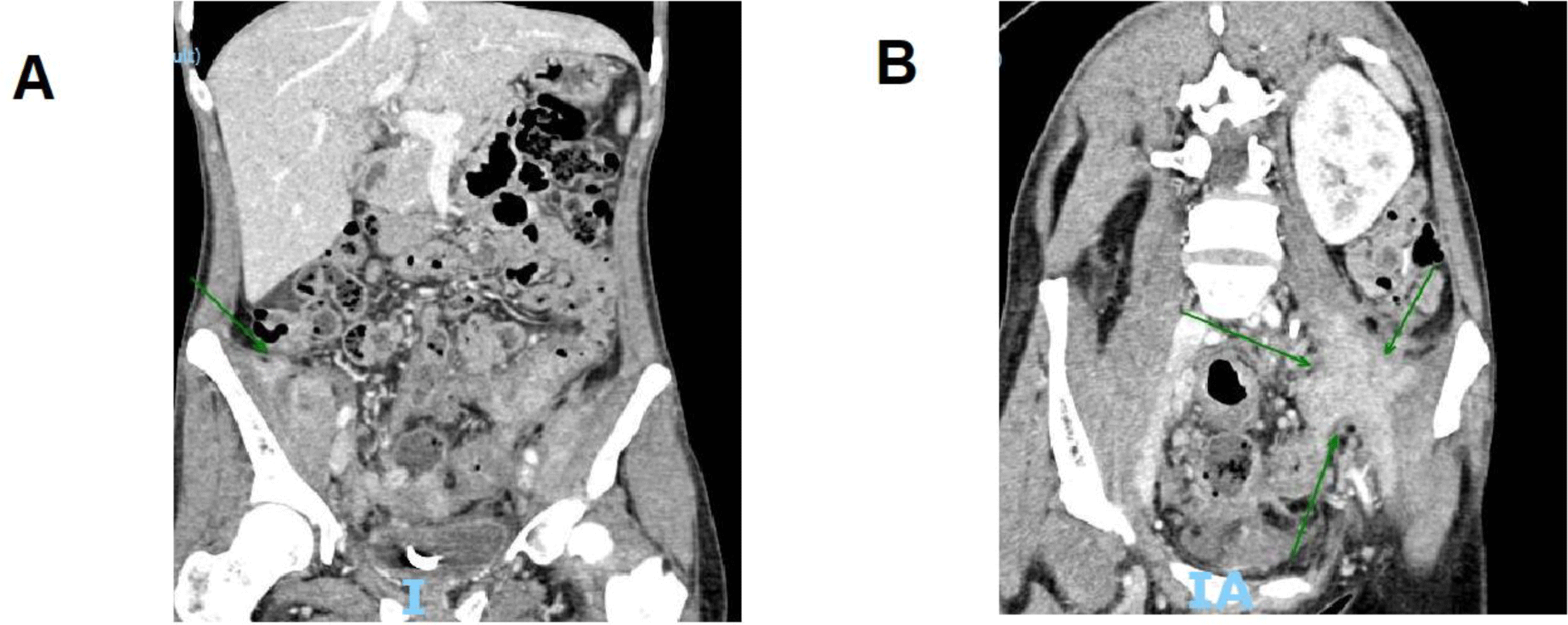
Upon examination, everything was normal except for both iliac fossae tenderness to palpitation and uncomfortable stretching by both legs. The laboratory test revealed anemia with Hb of 79.7 g/L and MCV of 76.1 fL, while other laboratory tests were unremarkable. The abdominal CT scan showed a 2 x 2 cm2 right iliopsoas mass with blurred border and heterogeneous density beside the old left one remained unchanged in size and the double-J stent (Figure. 2).
To determine the diagnosis, we decided to perform a third laparotomy. Two masses located on both psoas muscles were found with firm adhesion to the adjacent colon. We also found many solid lymph nodes lied along the aortic artery and the right colon with 1 to 3 cm in diameter. Because of the high risk of bowel perforation and bleeding upon removal, taking a biopsy was the final decision.
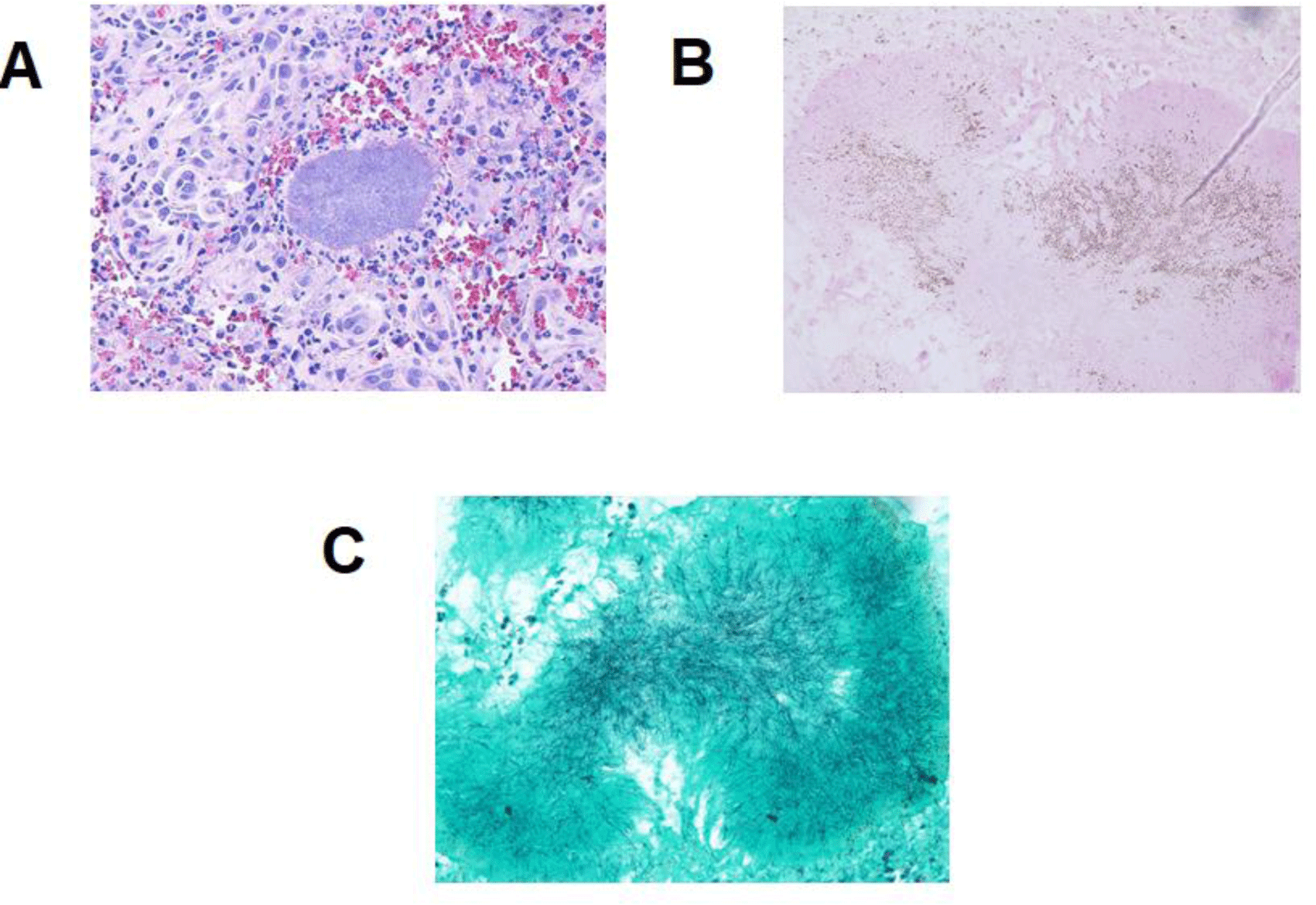
The diagnosis of actinomycosis in the psoas muscle was made by histopathology showing yellow sulfur granules composed of filamentous bacteria that are consistent with Actinomyces spp., a background of neutrophils after Gram, Grocott, and periodic acid-Schiff stain (Figure. 3).
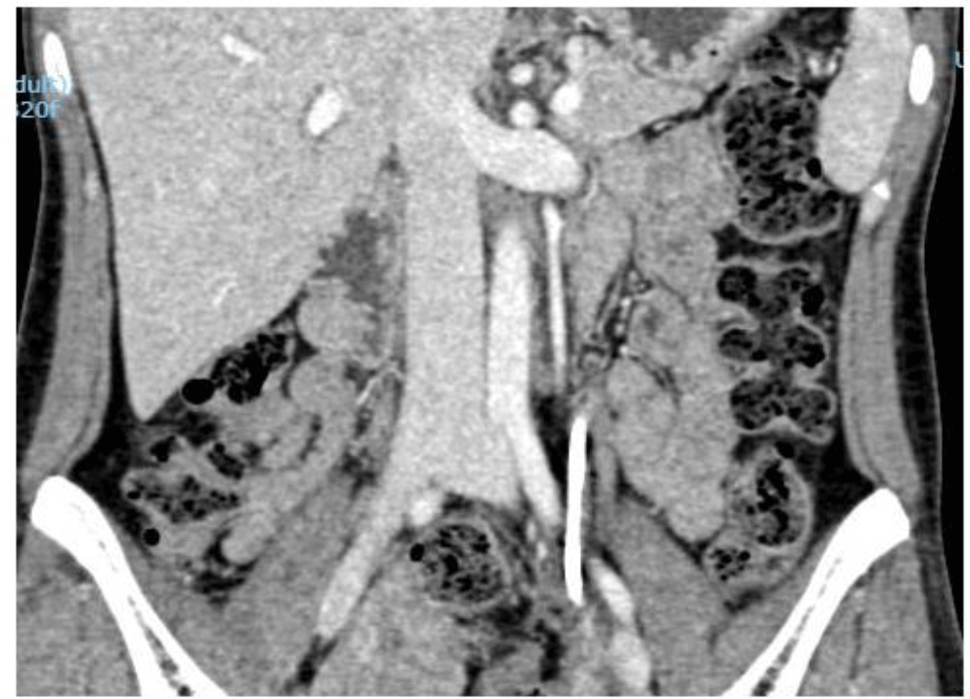
The patient was discharged with a two-week course of amoxicillin plus acid clavulanic 875/125 mg t.i.d and clindamycin 300 mg t.i.d. The first re-evaluation at an outpatient clinic showed a satisfactory outcome because her symptoms nearly disappeared. A three-month course of amoxicillin plus acid clavulanic 875/125 mg t.i.d was continuously prescribed. Upon the completion of the antibiotic therapy, both of the laboratory data (Hb 113 g/l) and abdominal CT scan showed a complete response (Figure. 4).
3. DISCUSSION
Actinomycosis is an uncommon chronic suppurative and granulomatous fibrosing infection caused by anaerobic Gram-positive bacteria, mainly within the Actinomyces genus [4]. In 1878, James Israel, a Jewish-German surgeon, described the first two cases of actinomycosis. Therefore, the most frequent causative agent of the actinomycosis, Actinomyces israelii (median 72.7% of cases), was named after him [4, 14]. The incidence of this infection was 1:300.000, reported in the 1970s, and the mortality varied between 0-28% [3]. In terms of pelvic actinomycosis, only 16 cases were reported from 1980 to 2014 in Asia [8]. This disease is dominant in developing countries, and threefold higher in men compared with women (except the pelvic form) [4]. In addition, middle-aged adults (30-50 years old) are more susceptible to this disease than other age groups [4, 6].
Actinomyces spp. are commensal organisms of the oral, gastrointestinal, and female genital tracts, with opportunistic infections occurring when the mucosal barrier is broken, leading to multiple abscesses and mass lesions [4, 5].
This disease occurs in three main clinical types: cervicofacial (40-60%), abdominopelvic (20-30%), and thoracic (10-20%), which occasionally overlap [4-6]. Abdominopelvic actinomycosis is a rare disease including abdominal infection, IUD-related pelvic abscesses, infections of the appendix, rectum, and liver [14]. Risk factors of actinomycosis include poor oral hygiene, alcoholism, the use of IUD, and some immunocompromised conditions such as diabetes mellitus and the use of immunosuppressive agents [13]. One report in Japan stated that more than 90% of women with pelvic actinomycosis had IUD [10]. In our case, the IUD was removed difficultly with four attempts which could have resulted in uterine mucosal injury. Two surgical procedures taken outside our hospital might facilitate the spread of Actinomyces spp. from the left psoas muscle to the right one. To the best of our knowledge, bilateral psoas abscesses caused by Actinomyces spp. has not yet been reported.
Diagnosis of actinomycosis can be challenged and frequently delayed because of the non-specific clinical signs. In abdominopelvic form, the initial symptoms may include vague lower abdominal pain, fever with or without palpable mass on examination. Other signs and symptoms depend on the site of the abscess, for example, hydronephrosis and difficulty mobilizing the hip in psoas abscess as were seen in our case and other cases [7, 11]. Imaging studies such as ultrasound and CT scan confirm the presence but cannot clearly distinguish benign from malignant tumors. Lymph node enlargement found during laparotomy at our hospital makes this case atypical in its presentation. Local or regional lymphadenopathy is unusual in actinomycosis [12]. Thus we could not rule out possibility of malignancy before having the histopathology result. Only 10% of cases are diagnosed preoperatively based on one report in 1985 [2]. Another report of 22 cases in South Korea from 2000 to 2006 showed that only one patient (4.5%) was diagnosed before surgery [5].
Definite diagnosis of actinomycosis relies on two methods: cultures or histopathology of the infected tissue [12]. Microbiological cultures are difficult, laborious, and negative up to 76% of cases [7]. The most reason is that Actinomyces spp. are very sensitive to oxygen therefore the specimen should be processed within a quarter-hour, immediately placed in anaerobic condition, and incubated in enough time (from 5 to 20 days) [4]. The histopathology of infected tissue is that the most utilized diagnostic method worldwide, which is mostly obtained after surgical intervention because of an initial misdiagnosis and usually positive in half of the cases [13, 14]. The presence of Gram-positive filament and sulfur granules might not be pathognomonic but are strongly suggestive of actinomycosis. Sulfur granules are colonies of bacteria that may be observed as distinctive patterns with basophilic clubs in the central part and eosinophilic clubs in the border on Hematoxylin and Eosin staining [4].
Regarding medical treatment, antibiotics remain the corner-stone. Fortunately, nearly all Actinomyces species are still sensitive to numerous antibiotics. Until now, there are no randomized control studies concerning antibiotic regimens, and most cases reported were treated based on expert’s opinions. The first choice of treatment would consist of a β-lactam and a β-lactamase. Doxycycline, clindamycin, and erythromycin are alternative agents in case of β-lactam allergy [4, 5, 7]. A prolonged treatment is mandatory to overcome the poor penetration of antibiotics into the fibrotic tissue and avascular planes. Conventionally, oral antibiotic therapy should be maintained for about 2 to 6 months in mild cases and 6 to 12 months for severe cases [1, 8]. For abdominopelvic form, surgery is reserved for complicated cases such as an extensive abscess or fistula tracts existence [4, 14]. In one Korean case series study, two patients with abdominopelvic actinomycosis underwent surgical resection and intravenous penicillin was administered for less than 4 weeks. Interestingly, none of them relapsed within the 34 months of follow-up [5]. Therefore, the role of surgery in abdominopelvic actinomycosis to reduce the antibiotic treatment time needs more studies.
Conclusion
Actinomycosis is an infectious disease that is usually missed in daily clinical practice. Actinomycosis should be suspected in any woman with the longstanding use of IUD who presents with a pelvic mass and/or abdominal pain. A prompt diagnosis and appropriate antibiotic treatment will improve outcomes and prevent the patient from unnecessary surgery.









