1. INTRODUCTION
Hepatic encephalopathy (HE) is considered as a brain dysfunction caused by liver insufficiency and/or porto-systemic shunting; it manifests with a wide spectrum of neurological and psychiatric symptoms and signs ranging from subclinical alterations to coma severely affecting the quality of life of patients and their caregivers [1].
HE is classified further into three types which are named A (acute), B (bypass) and C (cirrhosis). Firstly, type A HE is included in definition of acute liver failure characterized by a change in mental status, which may appear abruptly, fluctuate in severity and progress to deep coma. Its pathogenesis is caused by the astrocyte swelling leading to intracranial hypertension, cerebellar tonsils herniation and death [2]. Secondly, type C is the most common HE which pathologists frequently encounter it in clinical practice. It happens in cirrhotic patients resulting from porto-systemic shunting and liver failure which the latter plays the main role in pathogenesis because of failure of ammoniac detoxification [1, 2]. Finally, type B which is a very rare form of HE resulting from porto-systemic shunting, either in the complete absence of liver disease or in association with portal hypertension not due to cirrhosis. The diagnostic criteria for type B HE are identical to type C. However, type B HE is more often unsuspected or misdiagnosed given the absence of significant underlying liver disease and lack of awareness due to its rare prevalence [1, 2]. Here we report a case of type B HE due to a congenital superior mesenteric-caval shunt in the absence of liver cirrhosis and portal hypertension.
2. CASE PRESENTATION
A 57-year-old female presented to the hospital (University Medical Center, Ho Chi Minh City) with confusion and worsening cognitive impairment, displaying dyspraxia as well as inappropriate behavior. She had a background of well controlled hypertension and type 2 diabetes. She was a non-smoker and denied drinking alcohol. Her medications were Metformin 850mg and Losartan 50mg daily. There was no history of abdominal surgery or trauma and her family history was unremarkable.
On physical examination, she was not jaundiced and there were no signs of chronic liver disease nor any clinical evidence of portal hypertension. The patient’s weight was 61 kg, and her height was 158 cm making her BMI 24.4 which is regarded as overweight according to WHO Asian population classification [3]. Neurological evaluation revealed disorientation in time and place, ataxia and asterixis but no signs of neck stiffness, focal neurologic deficit or paralysis. The remainder of clinical examination was unremarkable. Full blood count and biochemical profile, including liver function tests were normal and INR was 1.2. Her serum ammonia level was elevated at 144 μmol/L (normal value range < 72 μmol/L). No evidence of a brain tumor or cerebral vascular disease was found on cranial CT scan.
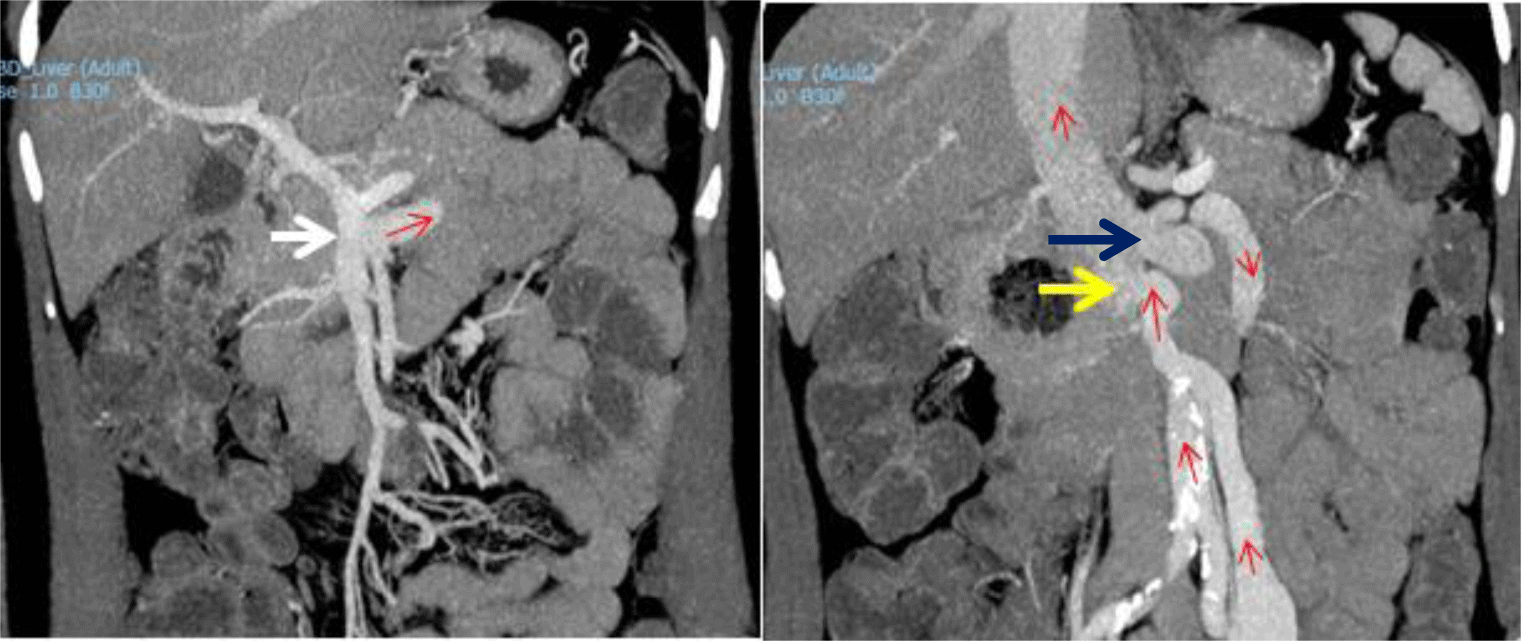
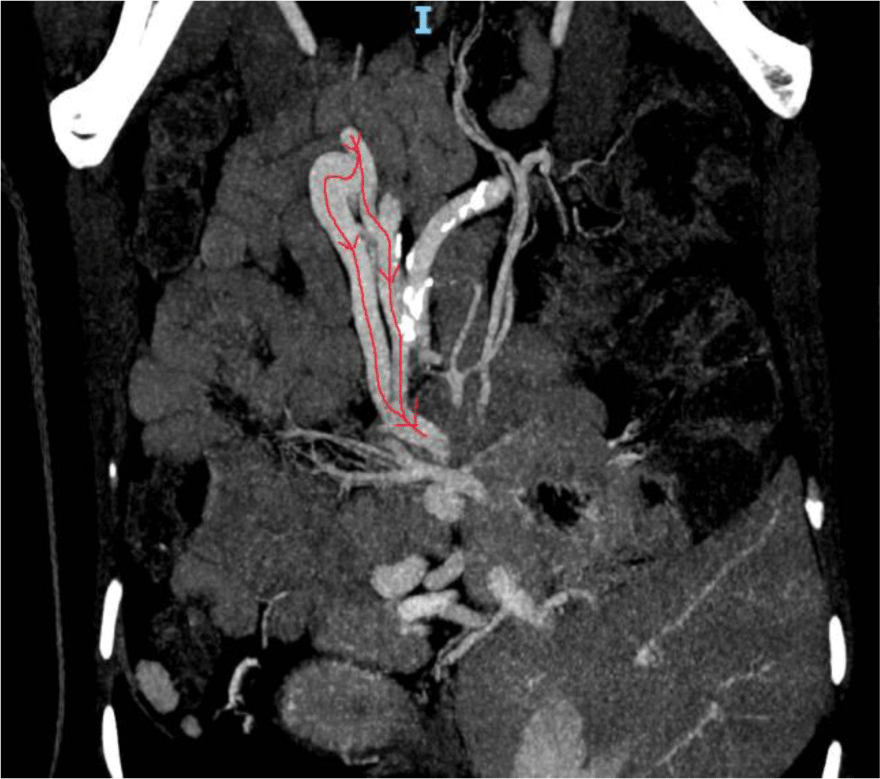
An abdominal CT revealed a superior mesenteric-caval shunt, consisting of a large tortuous vessel communicating between the superior mesenteric vein (SMV) and inferior vena cava (IVC) above the left renal vein (Figure 1). Abdominal Doppler ultrasound demonstrated blood flow from SMV to IVC through the shunt. Her liver elastography was F0 – F1, implying no significant liver fibrosis or cirrhosis. In conclusion, the definitive diagnosis was type B HE, caused by a large congenital superior mesenteric-caval shunt. Following radiological consultation and discussion with her family, it was decided to embolize the shunt with a written informed consent obtained from the patient.
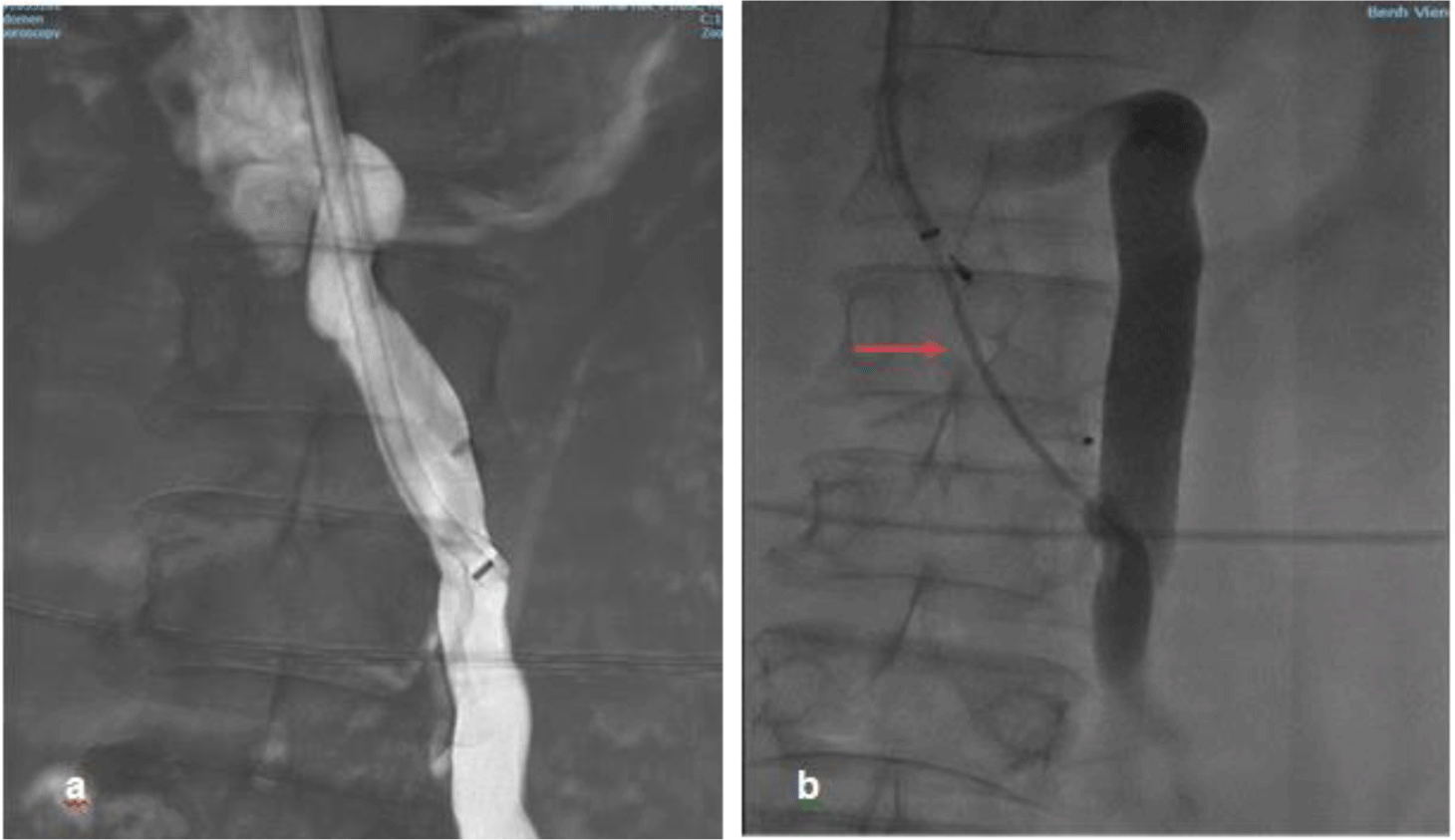
Venous access was obtained via the right internal jugular vein using ultrasound guidance under conscious sedation. The right hepatic vein was selected to evaluate the hepatic venous pressure gradient (HVPG). Wedge hepatic venous pressure, free hepatic venous pressure and HVPG were normal being 11mmHg, 6mmHg and 5mmHg respectively. The shunt was accessed under fluoroscopic guidance and venography was performed with the connection between the SMV and IVC below the left renal vein being confirmed and measured 11mm in diameter (Figure 2). A 16 mm Amplatzer vascular plug type II (St. Jude Medical, Inc. USA) was advanced and deployed across 2cm of the shunt near the IVC. Additional embolization with gelatin sponge was performed. Post-embolization venography demonstrated totally occlusion of the shunt (Figure 3)
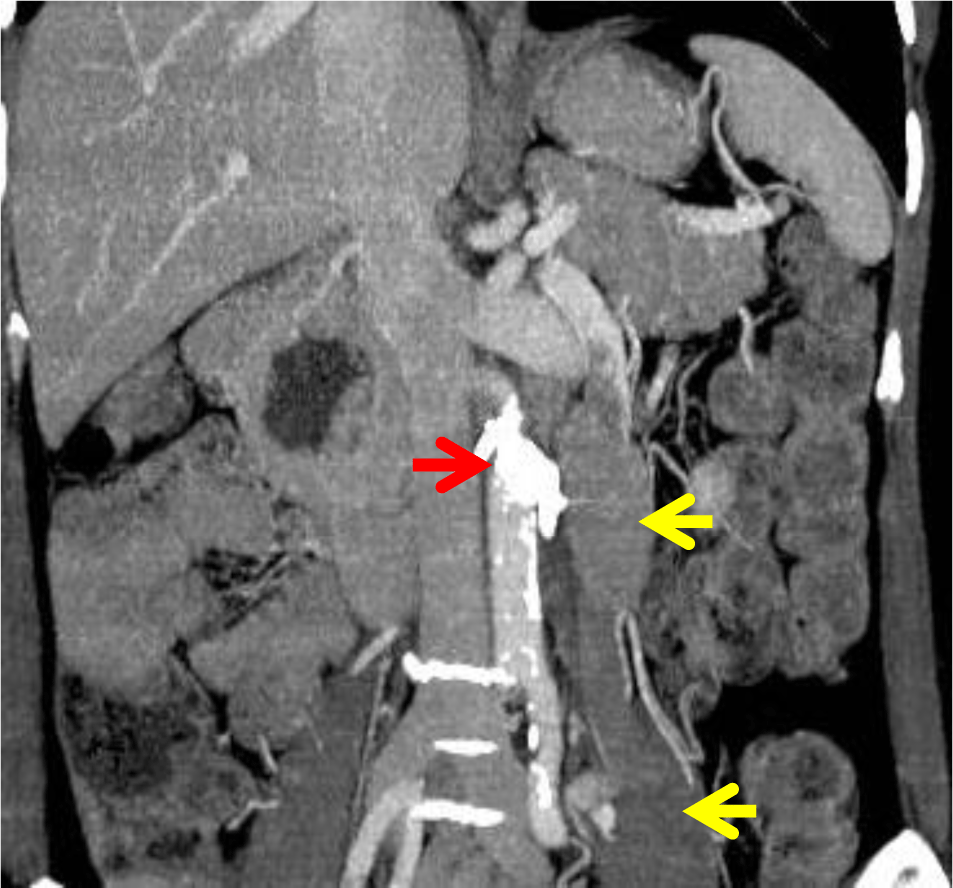
There were no complications and the patient’s serum ammonia level normalised within 24 hours of the procedure (63 μmol/L). A repeat CT scan on the following day showed successful shunt embolization (Figure 4). All neuropsychiatric symptoms resolved and at follow-up out to 9 months she has remained well, without any clinical evidence of encephalopathy and serum ammonia levels have remained normal.
3. DISCUSSION
Hepatic encephalopathy is a common complication of chronic liver disease of any cause. Type B HE is thought to result from the existence of communications between the portal and systemic venous systems, resulting in ammonia containing blood and other metabolites produced from the gastrointestinal tract easily bypassing the liver, proceeding straight into the venous systemic circulation thereby interrupting brain function [4, 5]. Extrahepatic collateral veins are most frequently observed in portal hypertension such as in liver cirrhosis or idiopathic portal hypertension [6]. In the absence of portal hypertension, extrahepatic porto-systemic venous shunting is extremely rare and is generally underdiagnosed because of lack of awareness. To our knowledge, there have only been 30 cases published, with the first report being in 1982 [7, 8]. The patient we describe did not have cirrhosis based on laboratory studies and imaging. Also, there was no clinical evidence of portal hypertension which was supported by the normal values obtained for wedge and free hepatic pressure. In addition, blood flow direction from SMV to IVC through this large tortuous shunt demonstrated by abdominal Doppler ultrasound helped to explain the persistent and recurrent hyperammonemia related neuropsychiatric manifestations. We therefore believe that our patient fulfills criteria for a diagnosis of type B HE.
The cause of the superior mesenteric-caval shunt remains unclear. While vascular communications without portal hypertension and liver cirrhosis can result from postoperative adhesions [9], our patient had not undergone any previous abdominal surgery nor experienced any abdominal trauma. It is therefore likely that the porto-systemic venous shunt was congenital. In the early stages of embryologic development, anastomoses exist between the subcardinal venous system and vitelline venous system [10-12]. The right subcardinal vein becomes part of the hepatic segment of the inferior vena cava, and the vitelline veins develop into the portal venous system and we believe our case represents a persistent communication between these two venous systems.
It is unclear why features of encephalopathy didn’t occur prior to the age of 57. However, other previously reported patients with encephalopathy due to a congenital porto-systemic anastomosis, also did not develop symptoms until later in life [13]. Possibly the symptom threshold may have been crossed as the shunt grew in size so that blood flow is preferentially diverted from the portal venous system into the lower pressure systemic venous one [14]. In addition, the aging brain may be less able to tolerate the presence of ammonia and other toxic metabolites [7]. Another possible explanation is that homeostatic control of the production of intestinal flora-derived ammonia may gradually become disordered with increasing age, resulting in the predisposition to hyperammonemia-induced encephalopathy with advancing age as has been proposed by Nishimoto et al. [13].
Chronic or recurrent disabling porto-systemic encephalopathy that is refractory to conventional treatment is generally considered an indication of shunt closure [2]. However, this decision must be balanced by risk/benefit considerations. In porto-systemic shunts associated with portal hypertension, the therapeutic blockade leads to overload of the portal venous system, increasing the risk for ascites formation and variceal bleeding [15]; portal pressure measurement before and after temporary occlusion of the shunt is therefore recommended to test tolerance to subsequent shunt closure [16-18]. In the present case, such risks were judged to be much lower as we carefully measured wedge and free hepatic venous pressure before and after temporary balloon blockage which showed no significant change in portal pressure. This demonstrated shunt occlusion did not significantly increase blood flow to the portal venous system. Additionally, our patient remains well on clinical and laboratory follow up after 9 months.
In the past, the only treatment option for extrahepatic porto-systemic venous shunts was surgical ligation [7, 19]. Recent advances in interventional radiological techniques have increased the option of transcatheter embolization. In the last two decades, this has now become the first line treatment. Techniques and devices are constantly evolving in the endovascular closure of congenital extrahepatic porto-systemic shunts including closure with coils [20] or a vascular plug [21]. Insertion of the Amplatzer vascular plug was minimally invasive and associated with a lower risk of device migration. It also proved effective successful shunt blockage. Furthermore, no major procedure related complications occurred.
Conclusion
In conclusion, type B HE due to a congenital extrahepatic porto-systemic shunt is an extremely rare condition that can be asymptomatic for a long period of time. Clinical manifestations are variable depending on its severity and comorbidities of the patient. The diagnosis is always challenging and in patients with unexplained, recurrent or progressive neuropsychiatric symptoms, without a defined cause, serum ammonia levels should be measured. When the serum ammonia concentration is raised in the absence of portal hypertension, an abdominal CT scan should be performed to exclude an extrahepatic porto-systemic venous shunt or related vascular anomaly.








