1. INTRODUCTION
Spontaneous hemopneumothorax (SHP) is a rare condition that is estimated to account for 1 to 12% of all cases of spontaneous pneumothorax [1]. In a case of massive hemorrhage, many patients can deteriorate quickly due to hypovolemic shock leading to a life-threatening condition. Prompt diagnosis and effective therapeutic intervention are required [2-5]. Initial management of SHP includes supportive and resuscitation measures with chest tube insertion, however, surgical approaches are indicated in patients with inadequate drainage [4]. Furthermore, video-assisted thoracoscopic surgery (VATS) is considered as an early treatment procedure to reduce morbidity [3]. In the present report, we reviewed our clinical experience of late recognition result in delaying surgical management for SHP.
2. CASE REPORT
A 21-year-old man with a three-hour history of sudden onset of left-sided chest pain was admitted to a district hospital. The patient reported no recent trauma. Posteroanterior chest X-ray demonstrated a small left-sided pneumothorax and a blunted left costophrenic angle (Fig.1A). The diagnosis in the district hospital was pneumothorax and pleural effusion was suspected due to ruptured lung tumor. Due to limited treatment capacity of the district hospital, the patient was transferred to a provincial hospital immediately in the Emergency Department at 1 AM on 26th April 2020. In the provincial hospital, on admission, his vital signs revealed a blood pressure of 110/70 mmHg, heart rate of 88 beats/min, and respiratory rate of 20 breaths/min. On physical examination, the patient had chest pain, no dyspnea. Hyper-resonant percussion and reduced air entry sounds were noted on the left side. Blood count on admission RBC: 4.66×1012/L, Hb: 140 g/L, HCT: 44.8%. Electrocardiography (ECG) showed normal sinus rhythm, biochemistry and coagulation tests were normal on admission. Thoracic computed tomography (CT) scans of the lung evidenced a left-sided hydropneumothorax (Fig. 1B). Ultrasound of the left thorax showed a large pleural effusion. The diagnosis in the emergency department of the provincial hospital was moderate pneumothorax and pleural effusion.
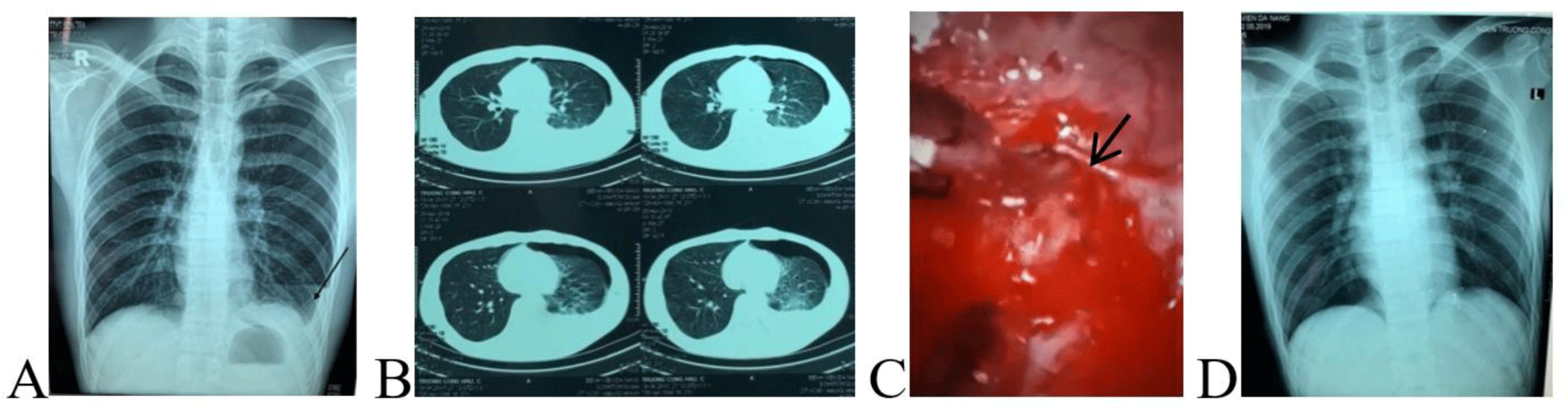
Then, the patient was referred to the Thoracic Surgery Department for chest tube insertion. At 10 AM (9 hours from the admission), while waiting for chest tube insertion, the patient had hypovolemic shock (blood pressure: 80/50 mmHg, heart rate: 82 beats/min, respiratory rate 28 breaths/min). After fluid resuscitation, the patient regained hemodynamic stability and emergency chest tube insertion was performed after nearly 12 hours from admission to the hospital. At the time of chest tube insertion, 1000 mL blood was drained with continuous bleeding, therefore, emergency VATS via three ports was performed. About 400 mL of blood and clots were evacuated from the left pleural cavity. We identified continuous bleeding from a small aberrant vessel at the top of the left thoracic cavity (Fig. 1C). Hemostasis was achieved via electronic coagulation. We could not find the air leak area. The postoperative course was smooth, no complications occurred. A postoperative chest X-ray after five days of VATS showed good expansion of the left lung (Fig. 1D) and the patient was discharged from the hospital one week after the operation.
At 9 PM, on 1st July 2019, a 62-year-old man was admitted to the Emergency Department of a provincial hospital because of ascending epigastric pain for two days. He denied any history of trauma, peptic ulcer, or pancreato-biliary disease. Based on his medical history, apart from epigastric pain, the patient was not accompanied by other symptoms. On admission, he was relatively hemodynamically unstable. His vital signs revealed a blood pressure of 90/60 mmHg, pulse rate of 112 beats/min, tachypnea, and body temperature 37.0oC. At physical examination, there were decreased breath sounds, as well as a dull percussion noted over the left lower chest. The abdomen was tympanums and there was epigastric tenderness.
His laboratory investigations showed leukocytosis (Full Blood count: WBC: 14.5×109/L, NEU: 78%, RBC: 3.67×1012/L, Hb: 110 g/L, HCT: 34.5%. Other results including urea, creatinine, amylase, AST and ALT were normal. The patients received IV 500 mL of 5% Glucose. At 10:20 PM, one and a half hour after admission, the patient was in shock with blood pressure 80/60 mmHg, and fluid resuscitation was continued. In the Emergency Department, the patient was diagnosed with pneumonia, pleurisy, pneumothorax, and massive pleural effusion. The differential diagnoses were perforated peptic ulcer disease and acute pancreatitis complicated with respiratory distress and sepsis.
After fluid resuscitation, the patient’s vital signs were stabilized as well as his symptoms were relieved. CT scan of the lung was done in the Emergency Department of provincial hospital demonstrated a large left-sided hydropneumothorax, hematoma, multiple apical bullae at the left upper lobe lung apex, and left lung collapse with a deviation of the mediastinum to the right side (Fig. 2A). Meanwhile, a non-contrast abdomen CT imaging showed no abnormalities (Fig. 2B).
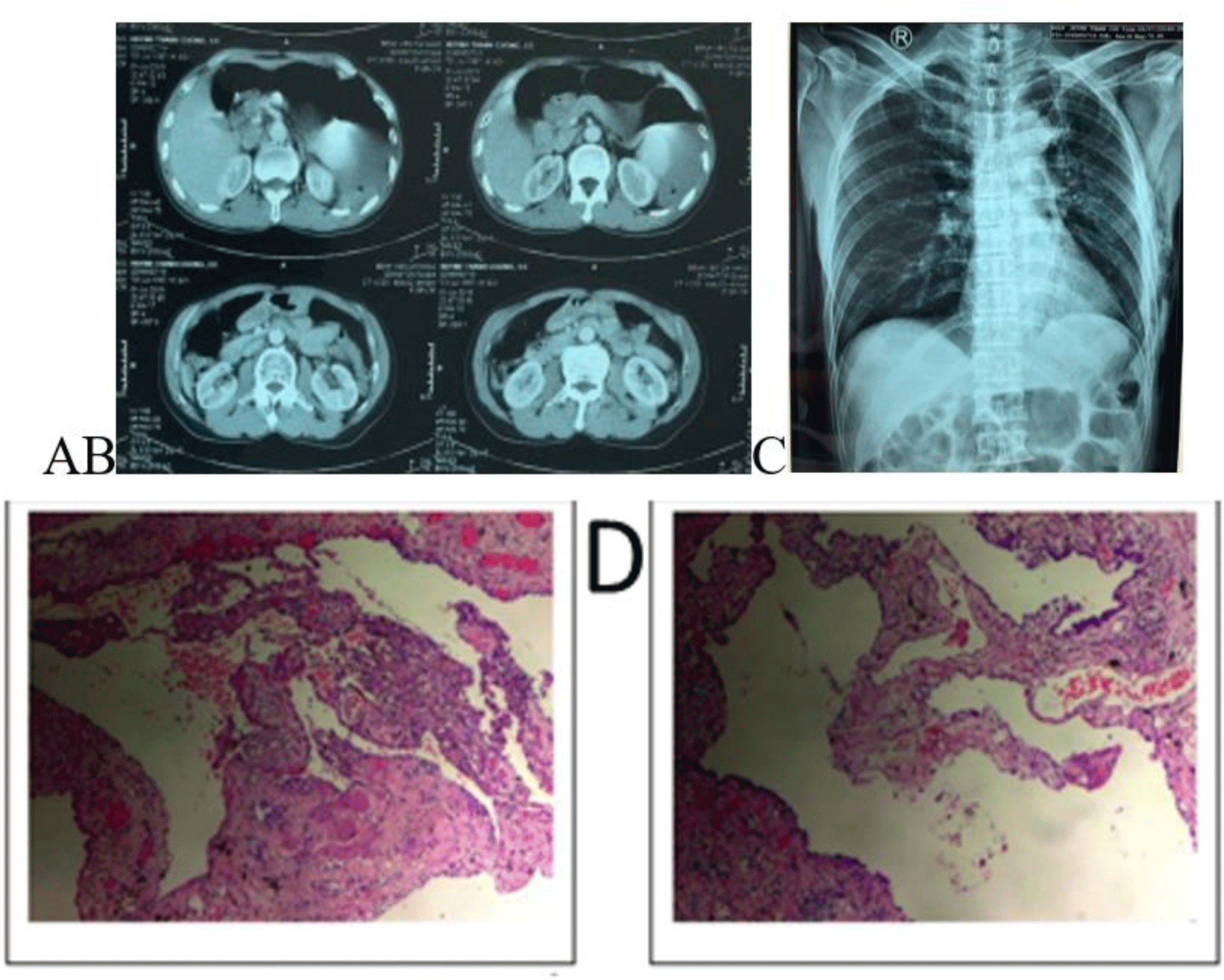
An emergency thoracostomy tube was inserted at 2:44 AM on 2nd July 2019, with an initial drainage of 2000 mL of blood and continuous bleeding. Instantly, emergency VATS was indicated to explore the left pleural cavity but VATS could not be performed due to excessive blood clotting in the pleura cavity and continued bleeding, thus the patient was transferred to urgent thoracotomy. During the operation, a lot of blood with clots were found inside the pleural cavity. We identified continuous bleeding from a small aberrant vessel at the top of the left thoracic cavity. Hemostasis was achieved via electronic coagulation. Bullae formations were resected by a linear stapler. Histopathological examination demonstrated a cystic lesion of the lung (Fig. 2D). During his postoperative follow-up, the patient was uneventful with no more epigastric pain. A postoperative chest X-ray showed good expansion of the left lung (Fig. 2C) and the patient discharged one week after admission.
At 2:50 PM, on 13 April 2020, a 48-year-old Chinese male was hospitalized at the Emergency Department of a provincial hospital with a three-day history of sudden onset of left-sided chest pain. His vital signs on admission were pulse: 92 beats/min, blood pressure: 140/80 mmHg, respiratory rate: 20 beats/min. By clinical examination, hyperresonant percussion and reduced breath sounds on the left chest were noted.
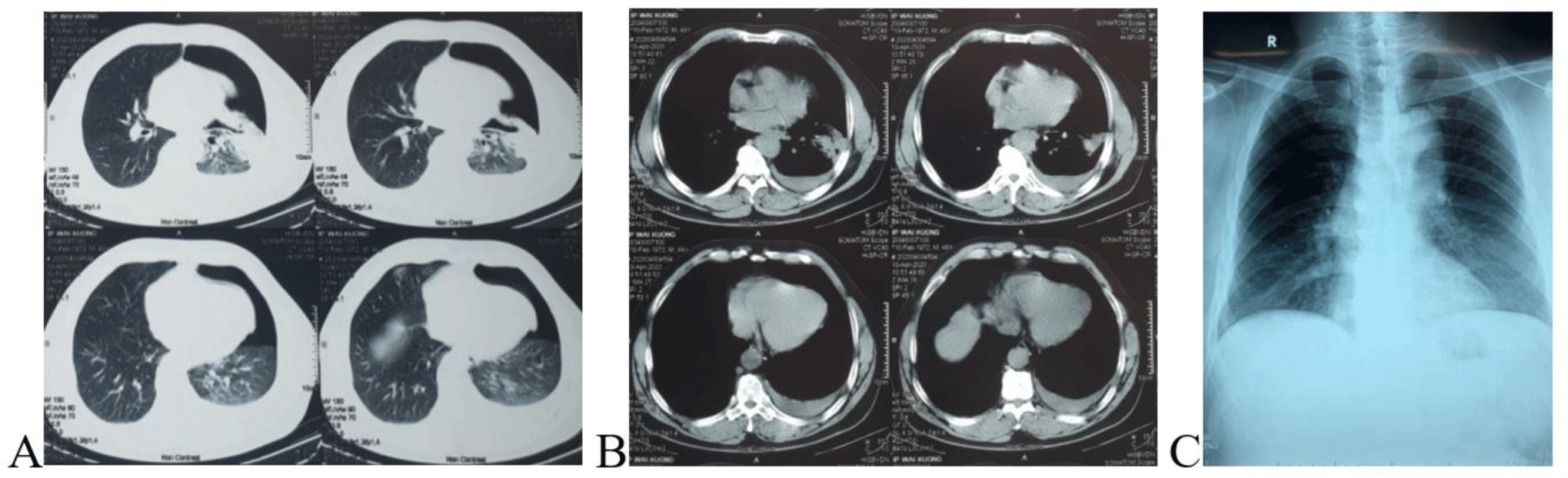
Laboratory tests were normal included full blood count, coagulation, blood glucose, urea, creatinine, serum electrolytes, and troponin. ECG showed sinus tachycardia, CT scan showed a large left-sided pneumothorax and small left-sided pleural effusion (Fig. 3A, B). Full blood count on admission and days after were shown in Table 1. Diagnosis on admission was pneumonia, pleural effusion, and pneumothorax.
| Date | Time | WBC (×109/L) | RBC (×1012/L) | Hb (g/L) | HCT (%) | PLT (×109/L) |
|---|---|---|---|---|---|---|
| 13 April | 3:58 PM | 8.67 | 4.92 | 149 | 44.4 | 194 |
| 13 April | 11:04 PM | 10.3 | 4.23 | 140 | 40 | 166 |
| 14 April | 5:47 AM | 12.02 | 4.37 | 133 | 39.6 | 157 |
After consulting with the doctor in the Thoracic Surgery Department, at 7 PM, a 32-Fr thoracic drain was inserted, and 300 mL of blood and air were evacuated immediately. Since his vital signs were stable, we continued conservative treatment with chest tube drainage and fluid infusion in the Intensive Care Unit and closely monitored the patient. During the following three hours, a further 1000 mL of blood was drained. The patient developed hemodynamic instability despite the aggressive fluid replacement. In view of the sustained hemorrhage, emergency VATS was indicated for hemostasis.
The patient developed hypovolemic shock (pulse: 100 beats/min, blood pressure: 80/60 mmHg) when arrived in the operative room. Instantly, the patient was resuscitated with fluid and blood products, at the same time VATS via three ports was performed for hemostasis at 11 PM (8 hours from admission). After entering the pleural cavity, about 300 mL of blood and clots were evacuated from the left pleural cavity. The bleeding point was identified from an aberrant vessel at the top of the left thoracic cavity, the bleeding site was controlled by electronic coagulation. We could not find the air leak site. Then, we inserted a chest tube and ports were closed.
After the operation, the patient was stable with a postoperative chest X-ray showing good expansion of the left lung (Fig. 3C). The patient was discharged five days after the surgery.
In all three cases, general anesthesia was performed using a single-lung ventilation technique with a double-lumen endotracheal tube. Demographics and clinical characteristics of 3 cases were summarized in Table 2.
3. DISCUSSION
SHP is a simultaneous accumulation of both air and more than 400 mL of blood in the pleural cavity without underlying trauma or any obvious cause. Progression of the clinical course can be dramatic and depends on the amount of air leakage and the volume of blood loss. In the case of massive hemorrhage, many patients can deteriorate quickly due to hemodynamic instability. It is found that sudden chest pain or dyspnea is the most common symptom for spontaneous hemopneumothorax [2, 3, 5]. However, a very slow progression of pneumothorax can result in the absence of respiratory symptoms. Even in rare cases, epigastric pain may be the first and most prominent symptom. Until now, the mechanism of epigastric pain in spontaneous hemopneumothorax is still uncertain. In the literature, epigastric pain might be elicited by tension pneumothorax or pleural effusion or highly collapsed lung toward pulmonary ligament that depressed the hemidiaphragm of the affected side [6]. In our study, the second patient was hospitalized with outstanding epigastric pain without respiratory symptoms. In our opinion, the absence of respiratory symptoms may be due to a very slow progression of pneumothorax, epigastric pain might be due to pleural effusion and a highly collapsed lung. Therefore, we recommend that a patient with epigastric pain, abdominal pathology should be screened for pneumothorax or pleural effusion. In spite of negative results on the abdominal examination, the clinicians should be aware of ischemic heart disease or spontaneous pneumothorax.
Chest X-ray and CT imaging are the most useful tools to diagnosis SHP and to assess the extent of hydropneumothorax, and the surrounding lung tissue [5, 7-9]. In most cases of SHP, chest radiographs demonstrated hydropneumothorax, while the remainder showed some opacity and obscurity of the costophrenic angle. Notwithstanding that the initial chest X-ray in approximate 10% of SHP patients may show pneumothorax only, with a radiological evidence of hemothorax developing later. Owing to the inconsistency between the X-ray image and the amount of drainage blood, there are some opinions that hemorrhage due to tube thoracostomy insertion, however, bleeding points can usually be confirmed intraoperatively. The reasons for the initial X-ray could not reveal hydropneumothorax may be due to the film being taken too early, a supine position rather than an erect/sitting chest film, or the possibility of delayed hemorrhage from a vascular adhesion band [4]. In our report, the first patient, the initial chest X-ray and CT scan showed a small left-sided hydropneumothorax which was inconsistent with blood drainage at the time of chest tube insertion. This might be due to the fact that films were taken at an early stage of the disease. Moreover, the normal blood counts could lead to misdiagnosis. Therefore, clinicians must be aware of spontaneous massive hemopneumothorax that may occur in patients who presented with ‘simple’ spontaneous pneumothorax or a small hemothorax accompanying spontaneous pneumothorax at the first instance.
The mechanisms of the bleeding can result from a torn adhesion between the visceral pleura and parietal pleura, rupture of vascularized bulla or underlying lung parenchyma or torn congenital aberrant or noncontractile vessels between the parietal pleura and bulla [2, 5]. The most commonly reported type of adhesion is the apical vascular adhesion associated with apical bullous disease. In all patients of our study, after entering the pleural cavity, we identified continuous bleeding from a torn small aberrant vessel at the top of the thoracic cavity. We suspected that the torn congenital aberrant vessels result from the progress of lung collapse which is developed mainly by an air leak from ruptured bullae. However, in the first and third patients, the site of air leaks from the lung surface was not seen by VATS. This may due to ruptured blebs causing pneumothorax that was sealed by clots. The initial management of SHP patients should be the insertion of tube thoracotomy and fluid resuscitation. It is considered that pressure hemostasis at the site of bleeding can be obtained by re-expansion of the collapsed lung after the insertion of a chest tube for hemopneumothorax, except for those due to vascular damage.
In the case of vascular damage-type hemorrhage, surgery is recommended in the early stage to reduce morbidity and mortality associated with continued hemorrhage and inadequate drainage [5, 10]. Emergency surgery management is indicated if the patients have (a) 1000 mL or more blood drainage at the time of chest tube insertion and continuous bleeding, (b) massive hematoma at computed tomography, and (c) clinical state of shock and/or more than 500 mL per hour in the first hour with 200 to 300 mL per hour subsequently. The need for elective surgery for SHP arises when complications relating to the SHP occur or if there is persistent air leak with pneumothorax [5, 9]. In our report, all three patients exhibited massive bleeding which can be leading to hypovolemic shock and life-threatening. In addition to fluid resuscitation and stabilization of vital functions with medical approach, emergency surgery was performed to hemostasis, hematoma evacuation, and resection of bullae.
Early intervention of SPH by VATS has been a popular recommendation in recent literature. The advantages of VATS over conventional thoracotomy include quicker access to the pleural cavity, a better view, more rapid manipulation, less postoperative pain, less blood loss, decreased need for blood transfusion as well as fewer post-operative complications and shorter hospital stays [3, 5, 11]. In our study, after fluid resuscitation, all three patients were hemodynamic stable, thus emergency VATS via three ports was performed.
Besides, VATS provides a good visualization of the pleural cavity and lung surface, VATS could identify the source of bleeding with the possibility to evacuate the blood clots and resection of bullae as well as pleurodesis [12]. In our case, VATS was easily implemented in the first and third patients. However, in the second patient, because of massive hematoma and hemorrhage, we were unable to access the source of bleeding. Thus, we promptly converted to open thoracotomy. Outcomes in our patients are consistent with previous reports [13, 14]. Despite the experienced surgeons, when using a thoracoscopic procedure, the presence of a hematoma sometimes obstructs the view and makes the approach of the bleeding point more difficult. In those cases, a change in the body position was effective. However, due to limited experience in the first period of VATS, the decision converted to open thoracotomy was an appropriate option in our condition. So delayed access to the source of bleeding could worsen the patient’s condition. For patients with SHP, we suggest that VATS is considered to be the first choice among surgical options, as the condition of bleeding from the torn congenital aberrant vessels is usually not too serious to control with VATS. On the other hand, it is easy to convert to a conventional thoracotomy in cases of necessity. The results of our study in accordance with previous studies, showed that VATS have many advantages over conventional thoracotomy (Table 3).
Conclusion
SHP is potentially a life-threatening condition and should be considered in male patients present sudden onset chest pain or epigastric pain accompanying hydropneumothorax on chest X-ray or abrupt onset hypovolemia signs with no apparent cause. Deterioration of patient with pneumothorax will be prevented by early diagnosis and treatment of SHP.
Early surgical intervention should be performed in patients who are hemodynamically unstable or show continuous bleeding from a pleural cavity drain.
VATS should be considered to be the first choice among surgical options in all patients with SHP, even those with active bleeding due to its effectiveness in reducing postoperative complications and facilitating rapid recovery.
Spontaneous hemopneumothorax is a rare emergency condition, estimated approximately 1 to 12% of all cases of spontaneous pneumothorax. Hypovolemic shock could develop and results in a life-threatening condition due to massive hemorrhage. The prompt diagnosis and management are required to achieve rapid hemostasis. A wide range of therapeutic intervention varities are considered to reduce morbidity and mortality such as supportive resuscitation measures, chest tube insertion, video-assisted thoracoscopic surgery.
In this study, we reviewed our clinical experience in the management for spontaneous hemopneumothorax. We identified possible causes and clinical presentation. Also, we recommend that early surgical intervention and VATS should be performed in patients who are hemodynamically unstable and with active bleeding.









