1. INTRODUCTION
Severe PHLF is a fatal complication, especially after major hepatectomy [1-5]. There are many criteria for selection of candidates for major hepatectomy [6, 7]. In Asia - Pacific region, Makuuchi’s criterion based on ICG clearance is usually applied to determine the extent of hepatectomy [8].
ICG clearance, through ICG-R15 is considered a standard to determine not only the indication but also the extent of hepatectomy, especially the major ones [6, 8-10]. ICG clearance is used to predict PHLF, especially after major hepatectomy [11, 12] and is more effective than Child-Pugh score and MELD score in pre-hepatectomy liver function evaluation [13]. However, many studies show that ICG-R15 is not reliable in predicting PHLF [2, 14] and has to be combined with other factors, especially remnant liver volume to eliminate major hepatectomy [4, 15-18].
With the development of radiology, we can calculate exactly the remnant liver volume [19, 20] which is mandatory in decision of majority hepatectomy [6, 21]. When the remnant liver volume is not sufficient, patients must undergo therapies such as pre-hepatectomy portal vein embolization (PVE) (with or without previous transcatheter arterial embolization: TACE) [22-25] or 1st phase of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) [26-28] for remnant liver hypertrophy.
Although there are many studies of combining ICG clearance, remnant liver volume, other clinical features to predict PHLF [4, 15, 16, 29, 30] but up to now, no standardized criteria of combining ICG clearance and remnant liver volume are confirmed to decide the extent of hepatectomy. Therefore, we carried out this study to analyze the effectiveness of combining ICG clearance and remnant liver volume to select major hepatectomy candidates as well as establish a model to predict PHLF.
2. MATERIALS AND METHOD
A prospective cohort study was conducted from October 2016 to March 2021 at University Medical Center - Ho Chi Minh city as one part of the research “The role of Indocyanine green test in evaluation of pre-hepatectomy liver function” which was accepted and allowed by the Ethical Board of Biomedical research of University of Medicine and Pharmacy at Ho Chi Minh City on July 9th 2019, ID 316/ÐHYD-HÐÐÐ.
All patients with ICG clearance and remnant liver volume before major hepatectomies for malignant and benign liver tumors or liver donation were included in the study excluding those with bile obstruction or chemotherapy within 1 month because these conditions influence ICG clearance test results. We selected 137 patients for this study.
Patients’ characteristics data, namely gender, age, and status of hepatitis, liver function tests, including the platelet count (PLT), international normalized ratio of prothrombin time (INR), total serum bilirubin, serum albumin (Child-Pugh score) were collected.
Pre-hepatectomy ICG clearance test was performed in all patients by LiMON method [31]. After intravenous injection of indocyanine green 0.5 mg/kg, the indocyanine green disappearance rate was calculated by linear regression from the plasma concentrations of indocyanine green at 5, 10, and 15 minutes that yielded two results: plasma disappearance rate (ICG-PDR) and ICG retention rate at 15 minutes (ICG-R15).
The remnant liver volume was evaluated on computerized tomography or magnetic resonance scan by manual method using software singo.via workstation from Siemens. The standard liver volume was calculated from the patients’ weight and height by Urata’s method [32]. The RLV/SLV ratio was used to decide major hepatectomy because this ratio is demonstrated more specific to predict PHLF than total function liver volume (FLV) [19]. Decision of major hepatectomy was based on usual criteria in which ICG-R15 and RLV/SLV were the most important [6, 8, 21]. When RLV/SLV was not sufficient, patients would undergo pre-hepatectomy procedures for liver hypertrophy such as PYE or 1st phase of ALPPS (with or without preceding TACE).
The operative factors, including the operation time, estimated blood loss, operative procedure and the post-operative factors, including histological fibrosis grading, tumor characteristics, liver function tests at post-operative day 3, 5, 7 (for PHLF diagnosis and grading), morbidity, mortality and hospital stay were recorded. Histological fibrosis staging was based on Ishak fibrosis staging system [33] for post-hepatectomy liver parenchyma (Ishak score). PHLF is diagnosed and graded by International Study Group of Liver Surgery (ISGLS) criteria, mainly based on the change of rerum bilirubin and INR on post-operative day 5 [34].
To avoid selection bias, we enrolled all patients who are satisfied study inclusion criteria. To minimize information bias, we used a standardized form to collect data, in addition to checking and retrieving as much as possible missing data from hospital files.
The patients’ data was analyzed using IBM SPSS 26.0 and R 4.0.5. Continuous variables were described by quartiles and compared by T-test and One-Way ANOVA test for standard distribution or Mann-Whitney U test and Kruskal-Wallis test for non-standard distribution. Nominal or ordinal variables were described by incidence and compared by Chi-Square test or Fisher’s Exact test. Post-hoc analysis between groups is by Tukey’s HSD method.
A model to predict PHLF was established by logistic regression model from multivariate model and reduced by backward stepwise variable selection based on AIC (Akaike Information Criterion). The model performance was validated and optimism corrected by 1000-time bootstrap resampling.
3. RESULTS
There were 137 patients including 113 men (82.5%) and 24 women (17.5%). The patients’ characteristics were shown in Table 1 and Table 2.
BSA: body surface area. INR: international normalized ratio. ICG-PDR: ICG plasma disappearance rate. ICG-R15: ICG retention rate at 15 minutes. RLV/SLV: remnant to standard liver volume ratio. RLV/P: remnant liver volume to weight ratio. TACE: transcatheter arterial chemoembolization. PVE: portal vein embolization. ALPPS: associating liver partition and portal vein ligation for staged hepatectomy
ICG-R15 in non-PHLF group was 0.73-time lower than PHLF group (95% CI was 0.56 - 0.95). ICG-DPR in non-PHLF group was 2.13% lower than PHLF group (95% CI was 0.37 - 3.89%). Serum albumin in non-PHLF group was 2.27g/L higher than PHLF group (95% CI was 0.40 - 4.15 g/L).
RLV/SLV in non-PHLF group was 1.13 -times higher than PHLF group (95% CI was 1.03 - 1.23) while RLV/P in non-PHLF group was 1.16-times higher than PHLF group (95% CI was 1.06 - 1.28).
Serum albumin was associated with the grade of PHLF (p = 0.046) but in post-hoc analysis, there was no significant difference between groups. With regard to remnant liver volume, only RLV/P had a significant difference of 1.17 times (95% CI was 1.02 - 1.33) between non-PHLF group and PHLF grade A group. Other differences were not significant.
There were 53 patients (38.7%) undergoing major hepatectomies with RLV/SLV under 40%. These patients had ICG-R15 median 4.30% (3.00 - 6.25%) and RLV/SLV median 35.00% (32.35 - 37.8%).
The incidence of PHLF in this group was 24.5% (13/53), tending to be higher than group RLV/SLV > 40% which was 11.9% (20/84) but the difference was not significant (p = 0.063). As regard the grades of PHLF, grade A was 13.2% (7/53), grade B was 11.3% (6/53), no grade C, also tending to be higher than group RLV/SLV > 40% but not significantly (p = 0.072).
If the 50-50 criterion was applied to diagnose PHLF, the incidence of group RLV/SLV < 40% was 9.43% (5/53), group RLV/SLV> 40% was 8.33% (7/84) but the difference was not significant (p = 0.527).
RLV/SLV in non-PHLF group tended to be lower than PHLF group but the difference was not significant (p = 0.601). ICG-R15 in non-PHLF group tends to be lower than PHLF group but the difference was not significant (p = 0.089).
| No-PHLF (n = 40) | PHLF (n = 13) | p (T-test) | |
|---|---|---|---|
| RLV/SLV (%) | 35.38 (32.50 - 37.81) | 33.82 (31.78 - 37.31) | 0.601 |
| ICG-R15 (%) | 4.05 (2.43 - 5.88) | 5.50 (4.20 - 8.05) | 0.089 |
Based on the demographic variables (gender, age) and pre-operative ICG-R15, Ishak’s histological fibrosis stage (Ishak score), remnant liver volume (RLV/SLV), two models for predicting PHLF after major hepatectomy were constructed using the logistic regression model:
-
- Model 1: multivariate of 5 upper variables
-
- Model 2: multivariate of 6 upper variables but reduced by backward stepwise variable selection based on Akaike Information Criterion (AIC)
Table 6 demonstrated estimated parameters from these models and their performance in predicting PHLF after major hepatectomy in which, old age, male, high ICG-R15, low RLV/SLV correlated to PHLF in univariate model. In multivariate model, only age and RLV/SLV remained the correlation to PHLF. This was presumably because the RLV/SLV was influenced by other factors including age, gender, ICG-R15, the effects of these factors were partially reflected by RLV/SLV. Ishak score was insignificantly correlated to PHLF and eliminated out of the reduced multivariate model.
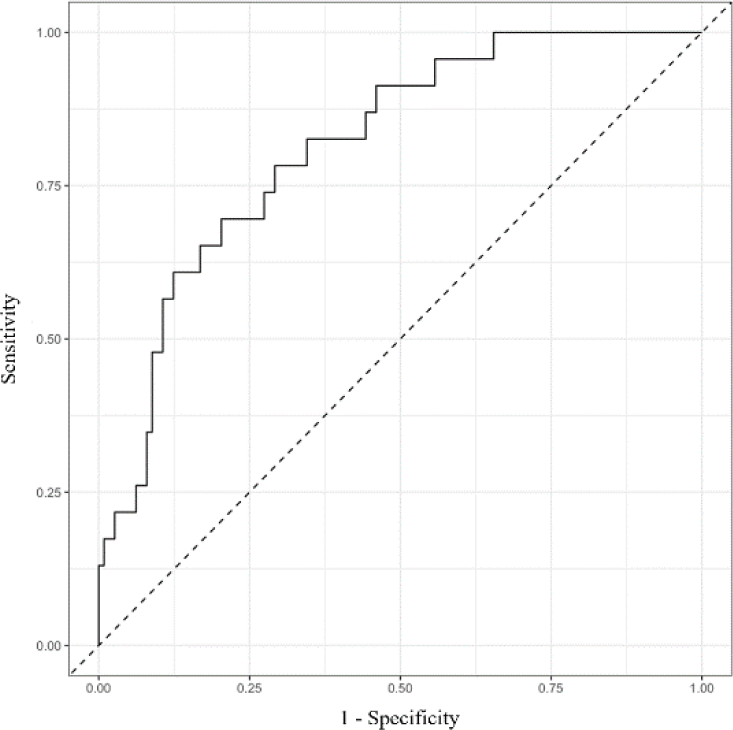
The model performance was validated and optimism corrected by 1000-time bootstrap resampling. The reduced multivariate model and the full multivariate one were equally effective with AUC = 0.77 (Figure 1).
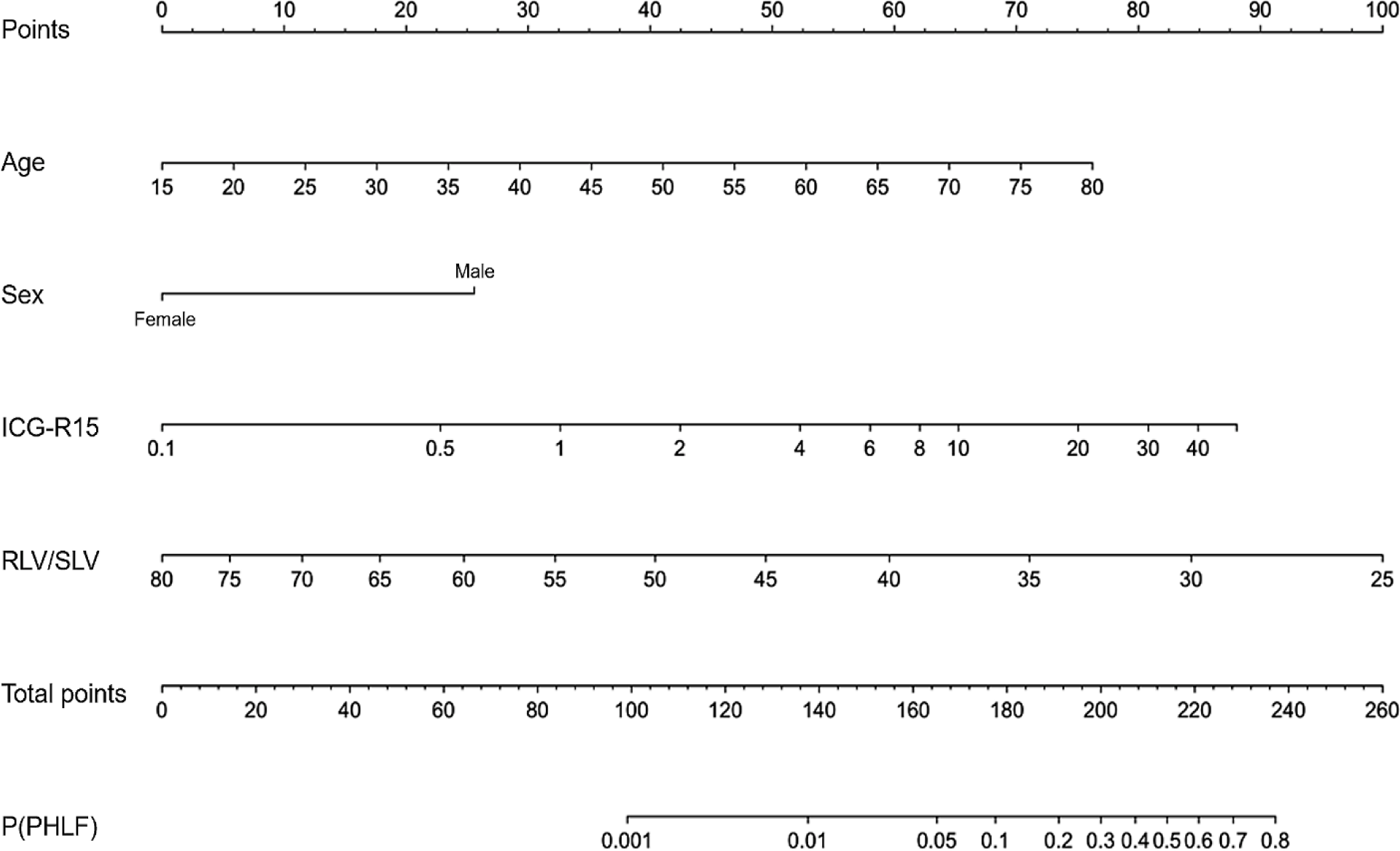
For clinical application, we can use the predicting equation for PHLF from the parameters (Table 7) or the simplified nomogram from the model (Figure 2).
| Parameters | Value |
|---|---|
| α (x-intercept) | 12.20683 |
| β1 | 0.07044 |
| β2 | -1.53612 |
| β3 | 0.58986 |
| β4 | -3.57920 |
We could use the parameters in Table 7 and age, sex (Female = 1, Male = 0), ICG-R15, RLV/SLV to calculate the probability of PHLF.
P(PHLF) = plogis(α + β1×Age + β2×Female + β3×log2(ICG-R15) + β4×log2(RLV/SLV))
We could simplify our process of calculation using the nomogram below: (1) calculate the points of every variable based on age, gender, ICG-R15, RLV/SLV and the total points, (2) calculate the probability of PHLF from the total points.
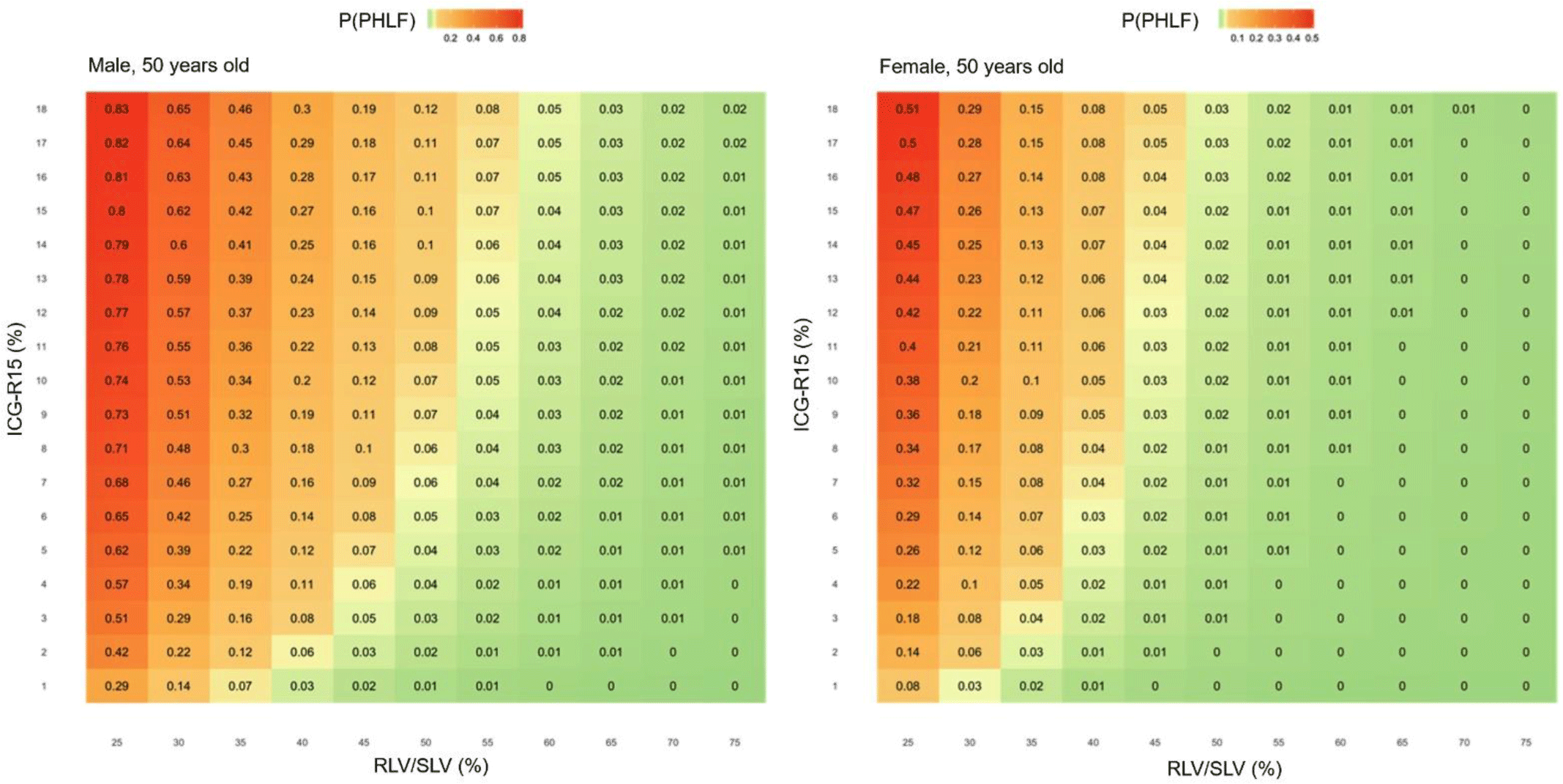
Figure 3 demonstrated the probability of PHLF for 50-year-old male and female with different values of ICG-R15 and RLV/SLV.
4. DISCUSSION
In this study, PHLF incidence was 16.8% in which grade B-C was 5.8%. This was quite low compared to other studies with PHLF higher than 20% [2, 4, 5, 15]. ICG-R15 in non-PHLF group was 0.73-time lower than PHLF group, which was similar to two other studies [12, 13].
Serum bilirubin, INR and platelet count was not significantly associated PHLF and its grades. Serum albumin, one of the liver function tests, affected by the nutrition, was significantly associated with PHLF with the difference of 2.27g/L. This was statistically significant but not clinically because the median serum albumin in both of non-PHLF and PHLF groups was in the normal range.
Histological fibrosis stage (Ishak score) was not significantly associated with PHLF and its grades. Int can be inferred that the effort to estimate the cirrhosis stage before decision of major hepatectomy was not important. Instead, ICG clearance, through ICG-R15 was more reliable in many pre-hepatectomy liver function tests to predict PHLF.
As regards the remnant liver volume, we used RLV/SLV mainly because this ratio was more specific in predicting PHLF [19]. RLV/SLV median in this study was 42.10%, corresponding to many recommendations for major hepatectomy in cirrhosis patients [22-24]. Both RLV/SLV and RLV/P in non-PHLF group were higher than PHLF group 1.13 and 1.16 times respectively. In PHLF group, RLV/SLV median was 38.63% (recommendation is 40%) and RLV/P median was 0.72% (recommendation is 0.8%).
The RLV/SLV among 3 groups non-PHLH, grade A and grade B-C were significantly different but the post-hoc analysis was not significant between groups. However, this suggests that the lower RLV/SLV, the more severe PHLF.
Notably, in this study, 53 patients, who were carefully selected based on ICG clearance test, underwent major hepatectomies with RLV/SLV < 40%. ICG-R15 median in this group was 4.30%. The incidence and grade of PHLH in this group tended to be higher than group RLV/SLV > 40%, but not significantly different.
In clinical practice, this result was noteworthy and consistent with medical literature. Surgeons could not estimate exactly the cirrhosis stage to decide the lowest value of RLV/SLV for each patient because the non-cirrhosis needs just 25%, compared 40% for the cirrhosis. This might lead to unnecessary procedures for liver hypertrophy which increases medical cost and prolongs the waiting time to operation.
Although the PHLF incidence tended to be higher than group RLV/SLV > 40%, the group RLV/RLV > 40% has no grade C PHLF and no mortality. If 50-50 criterion [35] was applied, this different would be insignificant too. This was a very important outcome of this study because this might help extend the indication of major hepatectomy with RLV/SLV lower than 40% and good ICG-R15.
Within group RLV/SLV < 40%, there was no significant difference of ICG-R15 and RLV/SLV between non-PHLF and PHLF groups. However, in non-PHLF group, ICG-R15 median tends to be lower than 5% and RLV/SLV tends to be higher than 35%. The model to predict PHLF after major hepatectomy would make the combination of these two factors clear and easy to apply.
Old age, male, high ICG-R15 and low RLV/SLV were associated with PHLF after major hepatectomy in univariate analysis. In multivariate model, only age and RLV/SLV retained the relationship. This was probably because the RLV/SLV was influenced by other factors including age, gender, ICG-R15. So, the effect of these factors was partially reflected by RLV/SLV. Ishak score was insignificantly correlated to PHLF and eliminated out of the reduced multivariate model as age, gender and ICG-R15 could reflect the liver parenchyma condition instead of Ishak score. One more time, the effort to estimate Ishak score before major hepatectomy was not important.
Lee et al [36] showed the relationship between RLV/SLV and ICG-R15 in which, we could calculate the minimum remnant liver volume based on ICG-R15 to prevent PHLF. But the ratios from that calculation were quite higher than RLV/SLV in our study. This difference could be explained by the development of surgical techniques by which surgeons could reduce surgical risk factors of PHLF so that they could reduce the remnant liver volume.
The model performance was 77% showing that this was quite good model and comparable to Honmyo et al (AUC 0.794) [15], Yamamoto et al (not using ICG-R15, AUC 0.796) [4]. One of the limitations of this model was that it could only predict the incidence of PHLF in total but not the grade B-C separately. This limitation was because there were just 8 patients of PHLF grade B-C in ISGLS criteria and 12 patients of PHLF in 50-50 criteria and we could not establish a model to predict PHLF due to this insufficient quantity. Another study with a larger sample size should be carried out with the need of at least 40-50 patients suffering PHLF.
Conclusion
The incidence of PHLF after major hepatectomy was 16.8% in which grade B-C was 5.8%. ICG clearance and remnant liver volume were associated with PHLF. With careful selection, patients with RLV/SLV under 40% and good ICG-R15 can undergo major hepatectomy safely. Based on gender, age, ICG-R15 and RLV/SLV, the incidence of PHLF after major hepatectomy could be predicted with the model performance of 77%.