1. INTRODUCTION
Eosinophilic gastrointestinal disorders are a group of clinical conditions that include eosinophilic esophagitis, gastritis, gastroenteritis, enteritis, and colitis [1]. These illnesses are characterized by eosinophilic infiltration of the gastrointestinal tract in the absence of other recognized causes of tissue eosinophilia [2]. Clinical symptoms differ from patient to patient, depending on the location and depth of eosinophilic infiltration into the gastrointestinal tract wall [3]. However, multiple ulcerative lesions are rarely reported [4].
Here, we reported an uncommon case of eosinophilic gastroenteritis and colitis presented with ulcers of varying diameter across the stomach, duodenal bulb, and colon.
2. CASE REPORT
A 57-year-old male presented to our hospital with chronic diarrhea. His symptoms started 6 months before presentation. He reported that he passed loose stool, 5 – 6 times a day with no blood or mucus in stool. He felt a burning sensation in the upper abdomen. He also had abdominal cramps. He lost 11 kg in 6 months (from 72 to 61 kg). The patient had no history of eczema, asthma, food sensitivity, or drug allergy. He did not contact patients with tuberculosis as well as patients with similar symptoms. He did not take any medication, particularly NSAID before symptom onset. He was a non-smoker. He lived in Tien Giang, a province of Vietnam and did not travel away from home recently. He denied a family history of inflammatory bowel disease (IBD). He was diagnosed with irritable bowel syndrome (IBS) at a local hospital and was treated with antispasmodic drugs and probiotics without any improvement.
At presentation, the patient was alerted and oriented, blood pressure was 120/70 mmHg, temperature was 37°C, heart rate was 80 beats per minute, and respiration rate was 14 breaths per minute. There was no skin rash, palpable peripheral lymph node, or pedal edema. The abdomen was not tender on palpation. The remainder of the clinical examination was unremarkable.
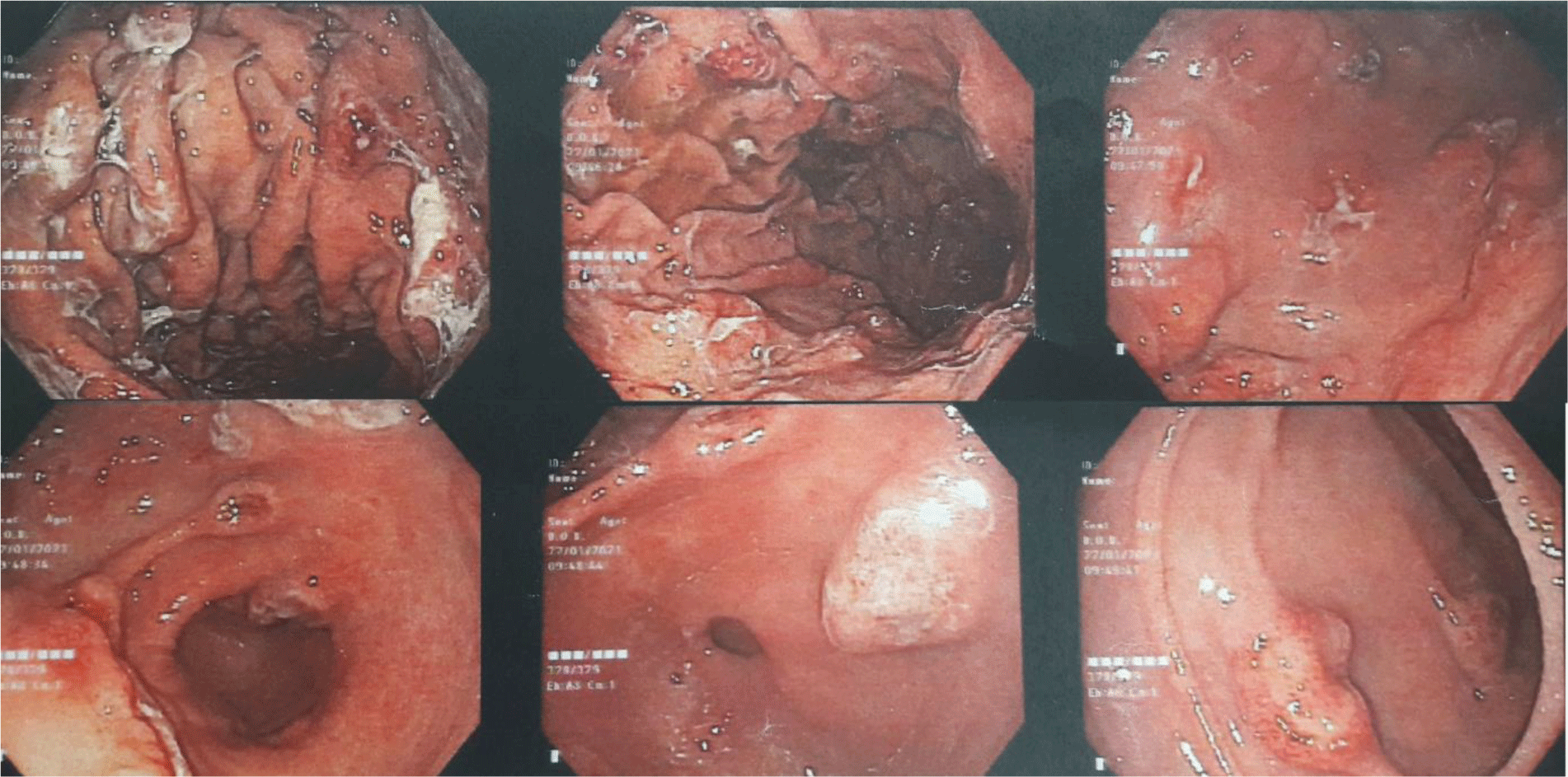
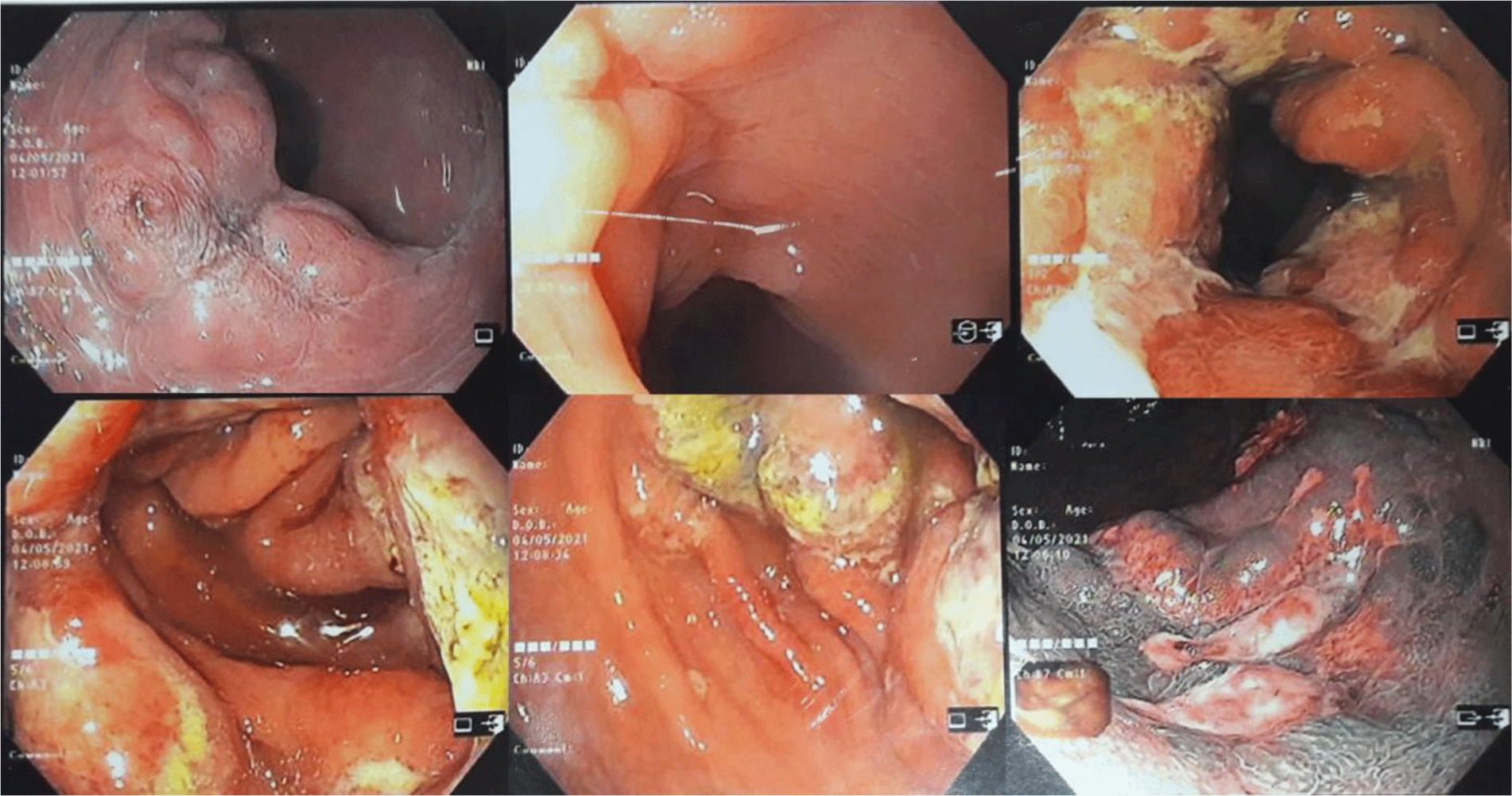
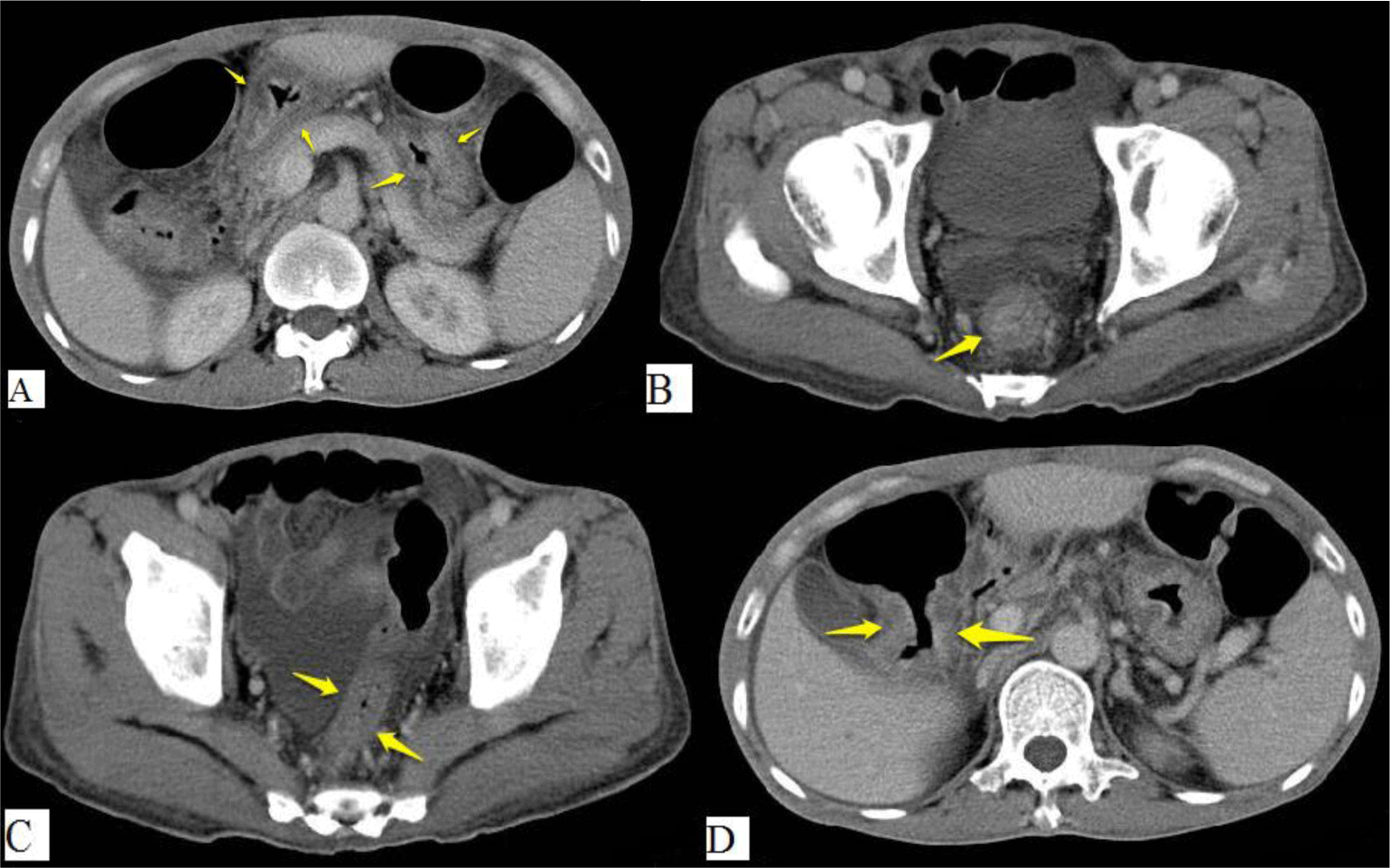
His complete blood count showed leukocytosis with marked eosinophil elevation of up to 15.8 x 109/L (white blood cell count of 23.27 x 109/L with 67.9% eosinophil, 19.7 % neutrophil, 10% lymphocyte). An increased amount of eosinophils with normal morphology were found in the peripheral blood smear. There were no other notable findings such as circulating blasts, atypical lymphoid cells, or dysplastic alterations. Biochemical tests showed the following information: CRP 3.2 mg/L (normal range < 6), albumin 3.3 g/dL (normal range 3.5 – 5.5), potassium 3.1 mmol/L (normal range 3.5 – 5.5), IgE 1335.6 IU/mL (normal range <100). Screening tests for autoimmune disease and vasculitis were performed. The test results were negative for antinuclear antibody (ANA), anti-double-stranded DNA antibody (anti-dsDNA), perinuclear anti-neutrophil cytoplasmic antibody (p-ANCA), and cytoplasmic ANCA (c-ANCA). Aminotransferase, blood urea nitrogen, serum creatinine, and thyroid function test were within normal limit. The patient had a normal echocardiogram. Stool specimen was positive for erythrocytes and leukocytes but without evidence of ova and parasite. Stool culture for common enteric pathogens was negative. Real-time PCR test for toxigenic Clostridium difficile was negative. Serology tests were positive for toxocariasis and Strongyloidiasis but were negative for Fasciola hepatica. The patient was negative for HIV. Bone marrow aspiration was performed. Eosinophils made up 48% of the bone marrow aspirate cellularity without aggregation or atypia. Background maturing trilineage hematopoiesis was present, but there was no dysplasia or increase in blasts. A hematologist was consulted to rule out hematologic disorders. An upper gastrointestinal (GI) endoscopy and colonoscopy were performed. Upper GI endoscopy showed thickened gastric folds with multiple ulcers of different sizes ranging from 5 to 20 mm at the fundus, body, antrum, gastric angle and duodenal bulb (Figure 1). Rapid urease test was negative. Colonoscopy showed ulcers from the rectum to the cecum with sizes ranging from 5 to 10 mm (Figure 2). The ileocecal valve was normal. Multiple biopsies were performed. Abdominal CT scan revealed thickened wall of the stomach, rectum, sigmoid colon, and ascending colon with ascites (Figure 3). Paracentesis could not be performed as there was too little ascitic fluid on abdominal ultrasonography.
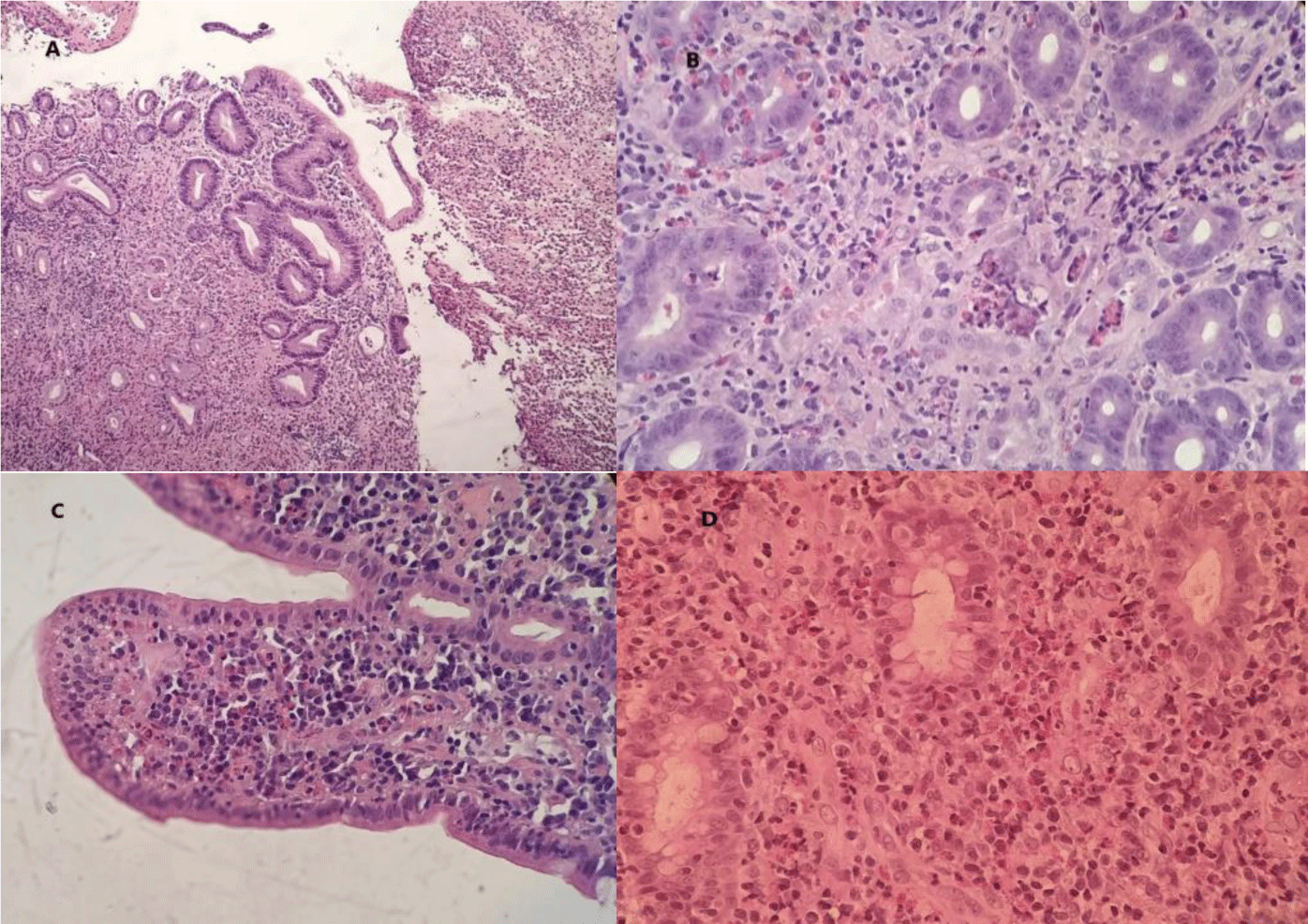
A course of Ivermectin 200 mcg/kg/day for 14 consecutive days was prescribed to treat suspected Strongyloides hyperinfection syndrome. However, the patient showed no improvement. The patient was then treated with Albendazole 400 mg twice daily for 5 days for toxocariasis but no symptoms amelioration occurred. Microscopically, the sections of stomach, duodenum and transverse colon showed ulcerated mucosa. Many eosinophils infiltrated the lamina propria and the glands. There were about 50, 55 and 90 eosinophils per high power field (HPF) in the stomach, the duodenum and the transverse colon, respectively. We did not find any features of granuloma, glandular distortion and parasite infection (Figure 4). Mucosal biopsy Mycobacterium tuberculosis polymerase chain reaction (PCR) assay was negative.
A course of proton pump inhibitor (PPI) pantoprazole 40 mg and IV methylprednisolone 40 mg were started. After 1 week of treatment, the patient symptoms resolved. A repeat complete blood count showed an absolute eosinophil count decrease to 5.61 x 109/L. The patient was discharged with oral methylprednisolone 40 mg a day for 3 more weeks. After that, corticosteroid was tapered (i.e. reduce dose by 4 mg/day at weekly interval). However, at dose of 20 mg methylprednisolone, the patient diarrhea reappeared. Therefore, azathioprine, a steroid-sparing agent was added to the regimen. Azathioprine was titrated to dose up to 2 mg/kg to maintain clinical remission. The plan was to repeat endoscopy and abdominal CT scan after 3 months of therapy. Unfortunately, our patient died of COVID-19 before that time mark.
3. DISCUSSION
Eosinophilic gastrointestinal disorders are rare inflammatory conditions [5]. In a population-based study conducted in the United States, the overall prevalence of eosinophilic gastroenteritis was found to be 5.1 per 100,000 persons, while that of eosinophilic colitis was found to be 2.1 per 100,000 persons [6]. Allergic disorders such as drug allergies, rhinitis, asthma, sinusitis, dermatitis, food allergies, eczema, or urticaria were found to be prevalent in patients with eosinophilic gastroenteritis or colitis [6]. The clinical manifestations of eosinophilic gastroenteritis and eosinophilic colitis are nonspecific and may mimic other gastrointestinal conditions such as functional dyspepsia or IBS [7, 8]. The patient in our case was misdiagnosed with IBS for 3 months. According to Klein’s classification, these disorders are divided into 3 categories: mucosal, muscular, and serosal subtypes [9]. Patients with a predominantly mucosal pattern common presentation include abdominal pain, nausea, vomiting, diarrhea, bleeding, anemia, protein-losing enteropathy, malabsorption, and weight loss. Patients with a predominantly muscular pattern are more likely to have pyloric or intestinal obstruction symptom. Patients with a predominantly serosal pattern present with eosinophilic ascites [1]. Our patient presented with a burning sensation in the upper abdomen and chronic diarrhea suggesting a predominantly mucosal pattern.
About 70% of patients with eosinophilic gastroenteritis present with peripheral blood eosinophilia. [5]. Other causes of peripheral blood eosinophilia such as parasitic infection, drug hypersensitivity, hypereosinophilic syndrome (HES), hematologic malignancy, and eosinophilic granulomatosis with polyangiitis (EGPA) must be excluded. Our patient had an absolute blood eosinophil count of (15.8 x 109/L). He was examined for parasitic infection by blood and stool tests. Empirical treatment for Strongyloidiasis and toxocariasis did not appear to show any benefit. A peripheral blood smear, bone marrow aspiration, and a hematology consultation were requested to rule out HES and other hematologic malignancies. EGPA was unlikely since this patient had no history of asthma, no signs of neuropathy or paranasal abnormalities, and his ANCA test results were negative.
In eosinophilic gastroenteritis, endoscopic manifestations may range from normal mucosa to erythema, white specks, focal erosions, ulcerations, fold thickening, polyps, nodules, and friability [8]. Eosinophilic gastritis primarily affects distal antrum and proximal small bowel. However, large and deep ulcers in the stomach are extremely rare [9]. T Kristopaitis and JS Scolapio reported 2 cases of gastroenteritis presented with a giant antral ulcer [10, 11]. N Katsumi et al reported a Japanese patient with numerous ulcerative lesions in the gastric body [4]. In our case, multiple ulcers of various sizes were found throughout the entire stomach and duodenal bulb.
In eosinophilic colitis, colonoscopy findings may include patchy areas of mucosal edema, punctate erythema, whitish elevated lesions, pale granular mucosa, and aphthous ulcer. These findings, however, are uncommon [8]. Our patient had multiple ulcerative lesions in every segment of the colon. To the best of our knowledge, this was the first reported case of eosinophilic colitis presented with large and multiple ulcers in the colon.
Histology is the gold standard for diagnosing eosinophilic gastroenteritis and eosinophilic colitis. Excess tissue eosinophilia in the absence of other recognized causes including parasitic infection, celiac disease, inflammatory bowel disease (IBD), drug hypersensitivity, connective tissue disorders, vasculitis such as EGPA, and HES is used as a diagnostic criterion[8]. Eosinophils reside in normal mucosa of the gastrointestinal tract. However, their distribution is not uniform along the bowel. In the colon, eosinophils are found more in the cecum and right colon than other segments. Normal tissue eosinophil counts range from < 10 eosinophils per high-power field (HPF) in the rectum to > 30 in the cecum [12]. The exact quantity of eosinophils needed to make a diagnosis is not well defined. In recent reviews, 30 eosinophils per HPF was proposed for the diagnosis of eosinophilic gastritis [13], whereas > 50 eosinophils per HPF in the right colon, > 35 eosinophils per HPF in the transverse colon, or > 25 eosinophils per HPF in the left colon were proposed for the diagnosis of eosinophilic colitis [8].
Corticosteroids remain the treatment of choice for eosinophilic gastroenteritis and eosinophilic colitis. A 2-week course of 20–40 mg prednisone per day has been shown to induce clinical remission in a majority of patients [8]. Symptom resolution in our patient occurred 1 week after the usage of methylprednisolone. However, his symptom relapsed during steroid tapering. Steroid dependency was reported to account for 20% of cases [5]. Azathioprine, an immunomodulator, has been shown to be effective in patients with steroid-dependent diseases [5, 8]. Our patient maintained his clinical response with azathioprine at a dose of 2 mg/kg.
The unexpected death of our patient made it impossible for us to evaluate the ability of azathioprine in inducing mucosal healing in eosinophilic gastroenteritis and colitis. However, we believe that the rare endoscopic manifestations of our patient make this case unique. Further clinical studies regarding the role of azathioprine for induction of endoscopic remission in eosinophilic gastritis and colitis are required.
Conclusion
In conclusion, we reported a rare case of multiple ulcerative lesions caused by eosinophilic gastroenteritis and colitis. It is critical to consider eosinophilic gastrointestinal disorders in the differential diagnosis of chronic diarrhea, particularly when patients presented with peripheral blood eosinophilia. Excess tissue eosinophilia in the absence of other causes is the key for the diagnosis of eosinophilic gastrointestinal disorders. Most eosinophilic gastroenteritis and colitis are corticosteroid responsive. In case of steroid dependency, azathioprine may be an option.









