1. INTRODUCTION
Pyoderma gangrenosum (PG) is a rare inflammatory, ulcerative cutaneous condition [1]. Typically, PG manifests as tender papules or pustules that progress to painful and rapidly expanding ulcers [2]. PG can be idiopathic or associated with a systemic disease such as inflammatory bowel disease (IBD), autoimmune arthritis, hematological malignancy, or solid malignancy [1]. The diagnosis and treatment of PG remain a great challenge [1]. In Vietnam, there are few studies regarding PG [3, 4]. In these studies, successful managements with corticosteroid were achieved.
Here, we reported a rare case of PG with ASUC which was successfully treated with oral cyclosporine.
2. CASE REPORT
A 34-year-old female presented to our hospital with hematochezia and multiple leg ulcers. A year before admission, she was diagnosed with moderate to severe ulcerative colitis which was treated with oral corticosteroid (i.e. 40 mg/day prednisolone for 2 weeks then gradually taper by 5 mg/day every week) in combination with mesalamine. Her ulcerative colitis was complicated by Clostridioides difficile infection for which she was treated with a 10-day course of oral vancomycin. Three months before admission, she stopped her ulcerative colitis medication due to the COVID-19 outbreak in Ho Chi Minh City, Vietnam. Two weeks before admission, she had bloody diarrhea six times a day and left lower quadrant abdominal pain. A week before admission, in addition to the previously mentioned symptoms, she had fever. Concomitantly, on both her legs, red, warm, painful pustules appeared and rapidly progressed to painful ulcers. With time, these ulcers increased in size. Therefore, she was admitted to our hospital.
At presentation, the patient was alerted and oriented, the blood pressure was 110/70 mmHg, the temperature was 37°C, the heart rate (HR) was 84 beats per minute, and the respiration rate was 15 breaths per minute. Physical examination revealed pallor of the conjunctivae and left lower quadrant abdominal tenderness. There were ulcers with undermined violaceous borders and surrounding erythema on both her legs (Fig. 1). The wound beds exhibited purulent exudates. On her left index finger, there was a tender pustule (Fig. 1). There was no palpable peripheral lymph node. The remainder of the clinical examination was unremarkable.
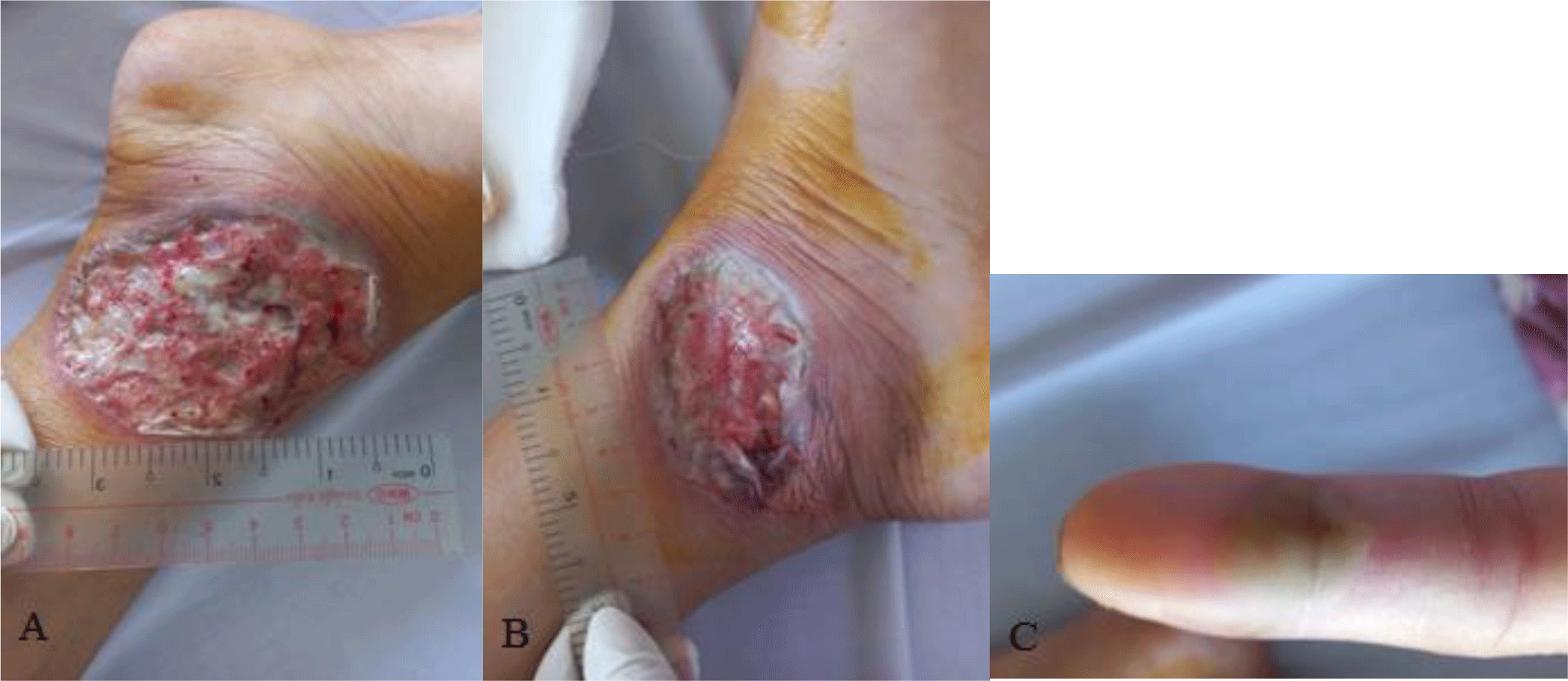
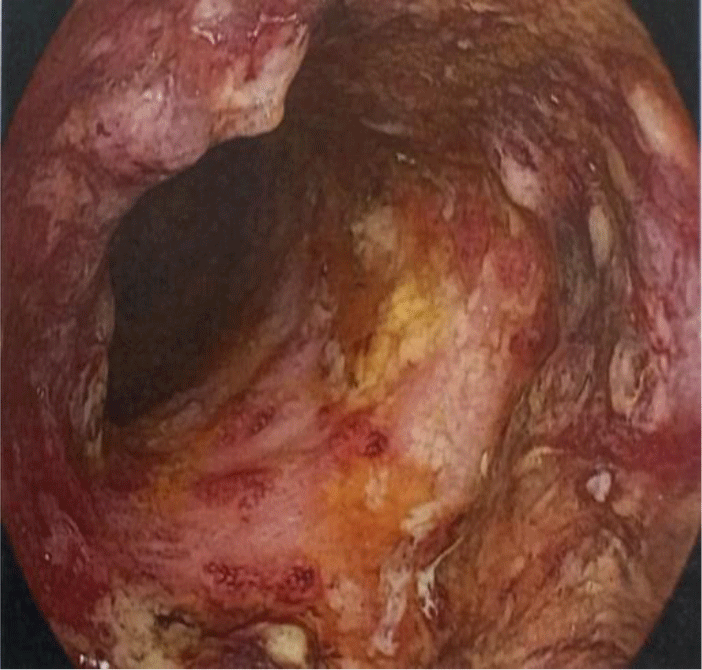
Her complete blood count showed leukocytosis (white blood cell count of 23.2 x 109/L with 82.7 % neutrophil), moderate anemia (Hemoglobin of 98 g/L), and thrombocytosis (platelet count of 509 x 109/L). Biochemical tests showed the following information: C-reactive protein (CRP) level of 102 mg/L, albumin level of 2.4 g/dL, potassium level of 3.1 mmol/L, magnesium level of 0.97 mmol/L, cholesterol level of 77 mg/dL. Aminotransferase, blood urea nitrogen, and serum creatinine were within normal limit. The patient had a normal chest X-ray. Serology test for HIV infection was negative. Sigmoidoscopy showed a loss of vascular pattern, marked erythema, friable mucosa, and ulcers suggestive of ulcerative colitis with a grade 3 Mayo endoscopic subscore (Fig. 2). Stool specimens were positive for erythrocytes and leukocytes. However, no ova and parasites were found. Stool cultures for common enteric pathogens were negative. Real-time polymerase chain reaction (PCR) test for toxigenic Clostridioides difficile was negative.
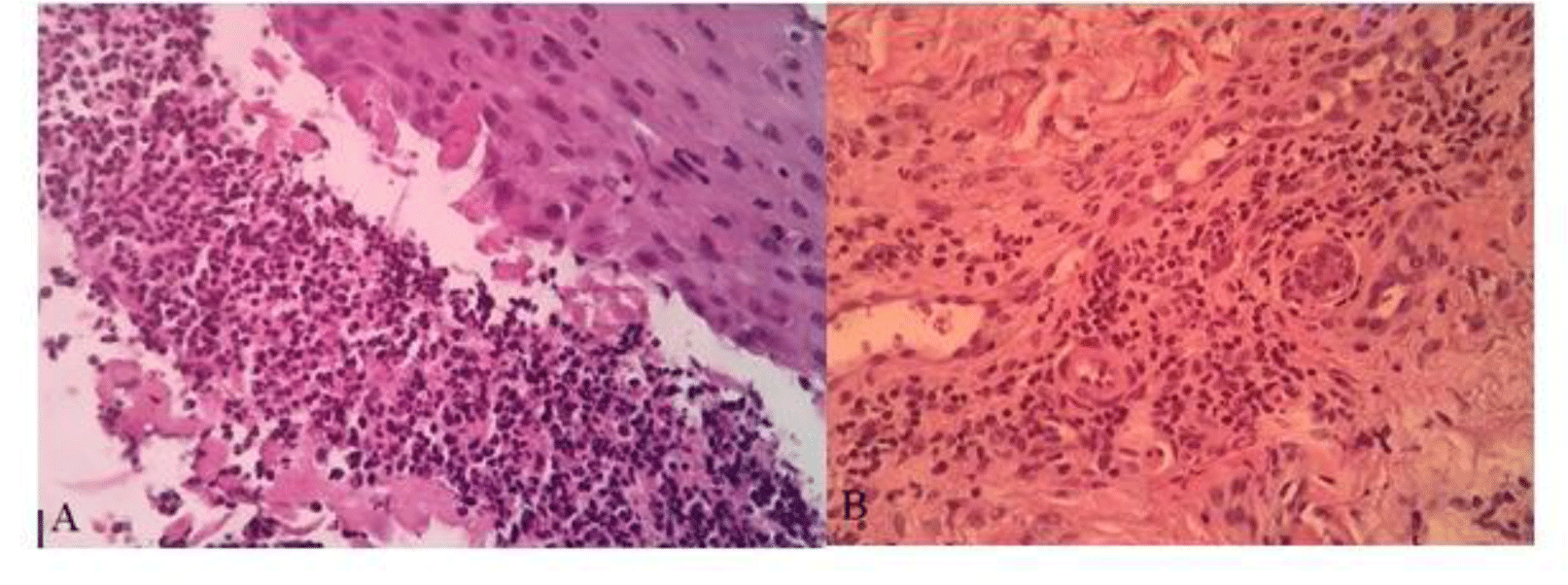
A diagnosis of ASUC and suspected PG was made and a dosage of 125-miligram intravenous methylprednisolone was started. As necrotizing skin infection could not be ruled out, broad-spectrum antibiotics with piperacillin/tazobactam and vancomycin were also introduced. Simultaneously, an extensive work-up for the diagnosis of her leg ulcers was carried out. On the third day of treatment, our patient still had three bowel movements a day with streak blood in her stool. Her leg ulcers did not show any improvements. Her CRP level was still high (60.1 mg/L). However, her pro-calcitonin level was low (0.1 ng/mL). Tests for autoimmune disease and vasculitis such as antinuclear antibody (ANA), anti-double-stranded DNA antibody (anti-dsDNA), perinuclear anti-neutrophil cytoplasmic antibody (p-ANCA), and cytoplasmic ANCA (c-ANCA) were negative. Antiphospholipid syndrome was also ruled out with negative Lupus anticoagulant, anticardiolipin IgM/IgG, and anti-β2-glycoprotein IgM/IgG. Doppler ultrasonography of the lower extremity arteries and veins showed no obstruction or thrombosis. Leg ulcer swabs for gram stain and culture were negative. A biopsy was performed at the border of the leg ulcer. The biopsy result showed epidermal neutrophilic infiltration and perivascular infiltrate composed predominantly of lymphocytes (Fig. 3). Based on the characteristics of the ulcers and pathological report, a diagnosis of PG was made.
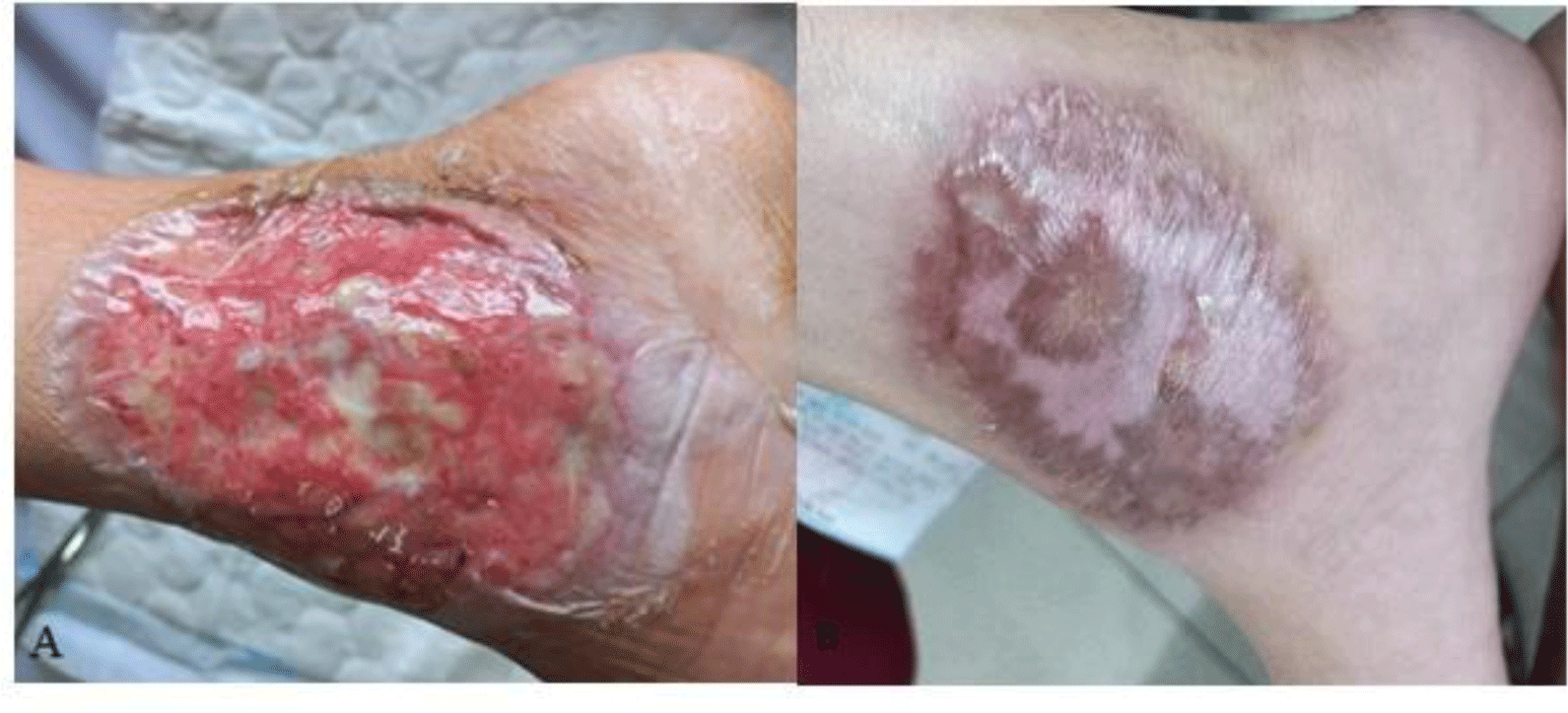
According to the Oxford criteria, our patient’s ulcerative colitis had no response to intravenous corticosteroid. Therefore, a rescue therapy with infliximab or cyclosporine was given for the patient. Our patient could not afford infliximab. Therefore, cyclosporine was the drug of choice. Cyclosporine could also be used to treat PG. Due to our patient’s hypocholesterolemia, oral administration was chosen. Cyclosporine microemulsion at a dose of 5 mg/kg was started and titrated to achieve a trough level of 150 – 250 ng/mL. Azathioprine was also introduced for maintenance therapy. Hydrocolloid dressings were used to improve wound healing. After a week of treatment, our patient’s bowel movement returned to normal with no blood in stool. The patient’s leg ulcers also showed sign of healing (Fig. 4). The patient was discharged and followed up at the Outpatient department. After 2 months, the ulcers resolved completely, leaving cribriform scars on both her legs (Fig. 4).
3. DISCUSSION
PG is a rare neutrophilic dermatosis. Its prevalence was reported to be 5.8 cases per 100,000 adults [5]. PG occurs in 1-2% of patients with IBD. Conversely, 36–50% of PG patients have IBD [6]. Our patient was diagnosed with ulcerative colitis a year before the diagnosis of PG. PG may or may not parallel IBD activity [7]. In our case, PG occurred when the patient had a severe activity of ulcerative colitis. Although PG can occur at any age, previous studies show that the average onset age is in the mid-40s [1]. The patient in our case was 34 years old at diagnosis. Nguyen Vu Hoang et al. described a previous case of PG with ulcerative colitis in a 21-year-old patient [4]. Shahana Shahid et al. also reported a case of a 22-year-old patient with PG and Crohn’s disease [8]. On the contrary, 45 idiopathic PG patients, reported by Tran Thi Huyen et al., had a mean age of 44.7 [3]. These data imply that PG may manifest at an earlier age when associated with IBD.
Historically, PG was a diagnosis of exclusion [1]. Our patient had pustules that rapidly progressed to painful ulcers with undermined violaceous borders. After the wounds healed, cribriform scarring was evidenced. Potential PG mimics such as antiphospholipid syndrome, venous stasis, vasculitis, skin malignancy, drug-induced tissue injury, and skin infection were also ruled out. Therefore, a diagnosis of PG could be made according to the criteria proposed by Su et al. [9]. This diagnostic approach was a challenging task. All other possible causes had to be ruled out before a diagnosis of PG could be established. Therefore, newer diagnostic tools for PG were developed such as the PARACELSUS score and the international consensus diagnostic criteria for PG [2, 10]. In the PARACELSUS PG diagnostic tool, major, minor, and additional criteria are assigned 3 points, 2 points, and 1 point, respectively. Patients with a score of 10 or higher are likely to have a diagnosis of PG [10]. With this approach, our patient had 16 points (3 major, 2 minor, and 3 additional criteria). Regarding the international consensus diagnostic criteria for PG, a biopsy of the ulcer edge demonstrating a neutrophilic infiltrate is required. Additionally, at least 4 minor criteria regarding histology, history, clinical examination, and treatment should be met [2]. Our patient had 7 out of 8 minor criteria. Patients with PG can be classified as mild or severe diseases based on the number, size, and location of their ulcers. Patients with multiple ulcers or a single ulcer with a size of 3 cm or greater, or an ulcer involving the face are classified to have severe PG [1]. Our patient had 2 large ulcers. Hence, a diagnosis of severe PG could be made.
ASUC is defined as the presence of bloody diarrhea 6 or more times a day and at least one sign of systemic toxicity including tachycardia (HR > 90/min), fever (temperature > 37.8°C), anemia (Hemoglobin < 105 g/L), or elevated inflammatory markers (erythrocyte sedimentation rate > 30 mm/h, or CRP > 30 mg/L) [11, 12]. Our patient had bloody diarrhea 6 times a day, anemia (Hemoglobin 98 g/L), and a high CRP level (102 mg/L). Thus, the diagnosis of ASUC was qualified. Intravenous corticosteroid is the mainstay of treatment for ASUC. Methylprednisolone 60 mg/day or hydrocortisone 100 mg 3 or 4 times a day is recommended [11, 12]. Corticosteroid could also be used to treat PG. In this case, the patient was given methylprednisolone at a higher recommended dose for ASUC as a concomitantly severe PG was suspected. Response of ASUC to intravenous corticosteroid is best assessed at day 3 of therapy [11]. The most widely used index to determine corticosteroid non-responders is the Oxford criteria. Patients with more than 8 bowel movements or 3-8 bowel movements accompanied by a CRP level above 45 mg/L had 85% probability of colectomy [12]. In non-responders, rescue therapy with infliximab or intravenous cyclosporine is recommended [11, 12]. At day 3 of treatment, our patient had 3 bowel movements and her CRP level was still high (60.1 mg/L). In this case, high CRP level could be explained by both conditions. However, if we waited too long to give rescue therapy, the patient could have a very high risk of colectomy. Therefore, rescue therapy was started. In the era of biologics, cyclosporine is not a preferred option due to its frequent therapeutic drug monitoring and its side effect profile. The adverse side effects of cyclosporine include nephrotoxicity, seizures, electrolyte abnormalities, hypertension, paraesthesia, gingival swelling, and opportunistic infections [13]. In this case, cyclosporine was chosen as our patient could not afford infliximab. In patients with ASUC, controlled studies showed that intravenous cyclosporine at dose of 2-4 mg/kg/day was useful [14].
Cyclosporine is a potent immunosuppressant. It binds to cyclophilin, an intracellular protein. The cyclosporine– cyclophilin complex binds to and inhibits the key phosphatase calcineurin. The inhibition of calcineurin suppresses T-cell proliferation [15]. Baseline renal function, liver function, blood pressure, serum cholesterol level, and magnesium level should be checked before cyclosporine administration [16]. The risk of neurotoxicity is increased in patients with hypocholesterolemia. If serum cholesterol level is below 3 mmol/L (105 mg/dL), cyclosporine should not be started or should be given orally (as the effect of low cholesterol level is related to the carrier in the intravenous preparation) [16]. Our patient had a low cholesterol level (77 mg/dL). Hence, oral cyclosporine was considered. Conventional preparation of oral cyclosporine has a low bioavailability [17]. It is not effective in inducing remission in patient with ASUC as ASUC requires a fast-acting drug. Cyclosporine microemulsion was developed to overcome absorption problem of the conventional oral formulation. An oral dose of cyclosporine microemulsion 5–6 mg/kg/day would be equivalent to 2 mg/kg/day of intravenous cyclosporine [15]. Uncontrolled study showed that microemulsion-based formulation of oral cyclosporine seemed to be effective in patients with ASUC [17]. Result of the STOP GAP trial, the largest randomized trial on PG, also showed that cyclosporine may have the same efficacy as prednisolone. In this trial, cyclosporine microemulsion at the dose of 4 mg/kg was used [18]. To treat both conditions, our patient was given cyclosporine microemulsion at dose of 5 mg/kg. Therapeutic drug monitoring is mandatory when using cyclosporine. A drug level of 150 – 250 is targeted (random levels for intravenous use, or trough levels for oral use) [15]. Wound care is also an important aspect in the management of PG. PG ulcers are often exudative [1]. Therefore, we chose hydrocolloid dressings. They absorbed exudate and provided a moist healing environment.
Anuraag Jena et al. reported a case of multifocal PG and ASUC. In this case, PG manifested as painful ulcerations in the perianal and calf regions. The patient responded to intravenous corticosteroid [19]. Magdalena Zychowska et al. also described a rare case of ulcerative colitis with vegetative variant of PG. Their patient improved with cyclosporine. In this case, the severity of ulcerative colitis was not mentioned. The authors also admitted that their patient was overtreated as superficial granulomatous pyoderma did not require aggressive treatment [20]. S Friedman et al. reported successful treatment of steroid-refractory PG with intravenous cyclosporine. The authors suggested that intravenous cyclosporine is the treatment of choice for steroid-refractory PG [21]. In Vietnam, Nguyen Vu Hoang et al. also reported a case of PG with ulcerative colitis. The patient was successfully treated with oral corticosteroid and mesalamine [4]. Our patient suffered from severe PG and severe ulcerative colitis concomitantly. Intravenous cyclosporine was required to control both condition. However, due to her low cholesterol level, oral cyclosporine was chosen instead. To the best of our knowledge, this is the first reported case of PG with ASUC successfully managed with oral cyclosporine in Vietnam. Oral cyclosporine could be an option to treat steroid-refractory PG and ASUC, especially in patients with limited treatment options.
Conclusion
In conclusion, we reported a rare case of PG associated with ulcerative colitis successfully treated with oral cyclosporine. PG should be included in the differential diagnosis of rapid progressive ulcers, especially in patients with IBD. Clinical characteristics and histopathological features are keys for diagnostic approach. Microemulsion-based oral cyclosporine may be an option for the management of steroid-refractory PG and ASUC, especially in patients with limited treatment options.








