1. INTRODUCTION
Chronic kidney disease (CKD) affects 10% of the global population. There were 3,171,000 patients undergoing renal replacement therapy worldwide in 2018, of which 2,823,000 received hemodialysis [1]. The primary types of permanent vascular access for hemodialysis include arteriovenous fistulas (AVF), arteriovenous grafts (AVG), and central venous catheters (CVC) [2]. AVF is the most commonly used. Annual Data Report of the United States Renal Data System on the Epidemiology of Kidney Disease spanning from 2012 to 2020 showed that AVF accounted for 63% of the hemodialysis patients, while AVG accounted for 16.7%, and various types of CVCs accounted for 19.6% [3].
Vascular complications presented in 15%–20% of end-stage renal patients hospitalised for routine hemodialysis [4]. Stenosis is the most frequent complication that accounts for 14%–42% [5]. Stenosis, occurring either at the anastomosis site or nearby, impedes the adequate flow of blood required for effective hemodialysis sessions and leads to local recirculation problems, thereby diminishing the quality of the hemodialysis procedure. Detecting stenosis at its early stages is challenging, especially when it affects the venous return. In addition to clinical examination and imaging diagnostics, the measurement of access recirculation (AR) by using urea-based method is another method employed to identify and assess the extent of stenosis. Numerous studies have been conducted to assess AR in patients receiving AVF treatment. These studies consistently demonstrate that refiltration phenomena are relatively common. For instance, in Beladi Mousavi’s 2010 study, recirculation was observed in 17% of cases, while Mamdouh Fakhry’s 2018 study reported a figure of 42% [6,7].
The prevalence of CKD is 12.8% in Vietnam, impacting around 8.74 million people [8,9]. Hemodialysis is the most common long-term treatment modality for individuals with end-stage renal disease patients in Vietnam [10]. However, few study has been conducted on the population of Vietnamese patients undergoing routine hemodialysis treatment for early detection of complications of AVF stenosis. Due to a lack of scientific evidence, medical practitioners cautiously use AR to screen and monitor hemodialysis patients with a high risk of AVF stenosis. Therefore, this study was conducted to assess the validity of AR in screening AVF stenosis in hemodialysis patients against duplex duppler ultrasound (DUS). The findings of this study provide useful evidence for medical practitioners to justify the necessity of prescribing AR to screen for AVF stenosis in hemodialysis patients with at high risk of this complication.
2. MATERIALS AND METHODS
This cross-sectional study was conducted at Cho Ray hospital. Eligible patients were those who had AVF hemodialysis for at least three months and consented to be a part of the study. The three-month minimum AVF maturity period was applied to minimize the likelihood of attributing any complications (if they arise) to the initial AVF surgery, ensuring that observed issues were more likely linked to the disease progression [11]. Patients who were unable to communicate or had health conditions requiring emergency care that hindered their participation were excluded.
The study was approved by the Ethics Committee for Biomedical Research, University of Medicine and Pharmacy at Ho Chi Minh City (320/HĐĐĐ-ĐHYD, 12th May 2020), and by the executive board of Cho Ray Hospital. All eligible patients were informed about the purpose of the study and invited to participate voluntarily. All included patients were asked to sign a written informed consent before participation. This report followed the STARD guidelines for reporting diagnostic accuracy studies [12].
The researchers gathered data from the patients’ clinical profiles and medical backgrounds, including age, gender, body mass index (BMI), the presence of hypertension, the presence of diabetes mellitus, the number of dialysis years, any prior placement of CVC, and history of previous AVF creation.
Experienced nephrologists performed physical examination, which encompassed several aspects. This included a thorough inspection of the AVF arm to check for any signs of swelling and the presence of collateral veins in areas of vasculature associated with the fistula arm, including the chest and neck. Additionally, two specific tests were performed. The arm elevation test was considered positive if the AVF did not collapse when the arm was raised above the level of the heart. The pulse augmentation test involved completely blocking the AVF several centimeters downstream from the arterial anastomosis with one hand while the other hand assessed the quality of the pulse. A positive result for the pulse augmentation test indicated that the pulse did not strengthen when the vein was occluded [13].
Cardiovascular ultrasonography specialists scanned all participants using DUS with a 7.5 MHz probe and the Winno E10 color flow duplex machine for mapping the AVF. AVF stenosis was identified when there was a reduction of at least 50% in the inner diameter compared to the adjacent vessel on the inflow side [14].
The calculation of access blood flow (Q) was performed using the formula: Q = Cross-sectional area (cm2) × minimal velocity (cm/s) × 60, with the cross-sectional area (cm2) being determined by π d2/4 (where “d” represents the diameter) [15]. Access blood flow was assessed at four distinct locations along the AVF, including the brachial artery supplying the AVF (Qa), the anastomosis, as well as 5 cm and 10 cm away from the anastomosis. The minimal blood flow value among these four sites was denoted as Qmin.
Three Blood Urea Nitrogen (BUN) samples were taken after approximately 30 minutes of treatment and after turning off ultrafiltration that follows the standard protocol:
-
Set the pump speed to 500 mL/minute (or maximum achievable rate).
-
Draw the arterial (A) and venous (V) line samples.
-
Immediately reduce the blood flow rate to 120 mL/minute.
-
Turn the blood pump off exactly 10 seconds after reducing the blood flow rate.
-
Clamp the arterial line immediately above the sampling port.
-
Draw the systemic arterial sample (S) from the arterial line port.
-
Unclamp the line and resume ultrafiltration and dialysis.
BUN in A, V, and S samples were measured. Then AR was calculated based on the following formula was applied: AR (%) = 100 × (S – A) / (S – V), where “S” indicated the concentration of BUN in the peripheral vein, “A” in the arterial line, and “V” in the venous line during hemodialysis. Blood samples were collected after reducing or halting the dialysate flow at the end of the dialysis session. It was crucial to confirm that the needles were correctly positioned and that the lines were not reversed before withdrawing blood for BUN measurements [16].
Fig. 1 describes study process. Once data collection was completed, the parameters which were described in (2) and (3) for both the AVF stenosis and non-stenosis groups were analysed. AR values (4) were used to screen for AVF stenosis<50% and AVF stenosis≥50%.
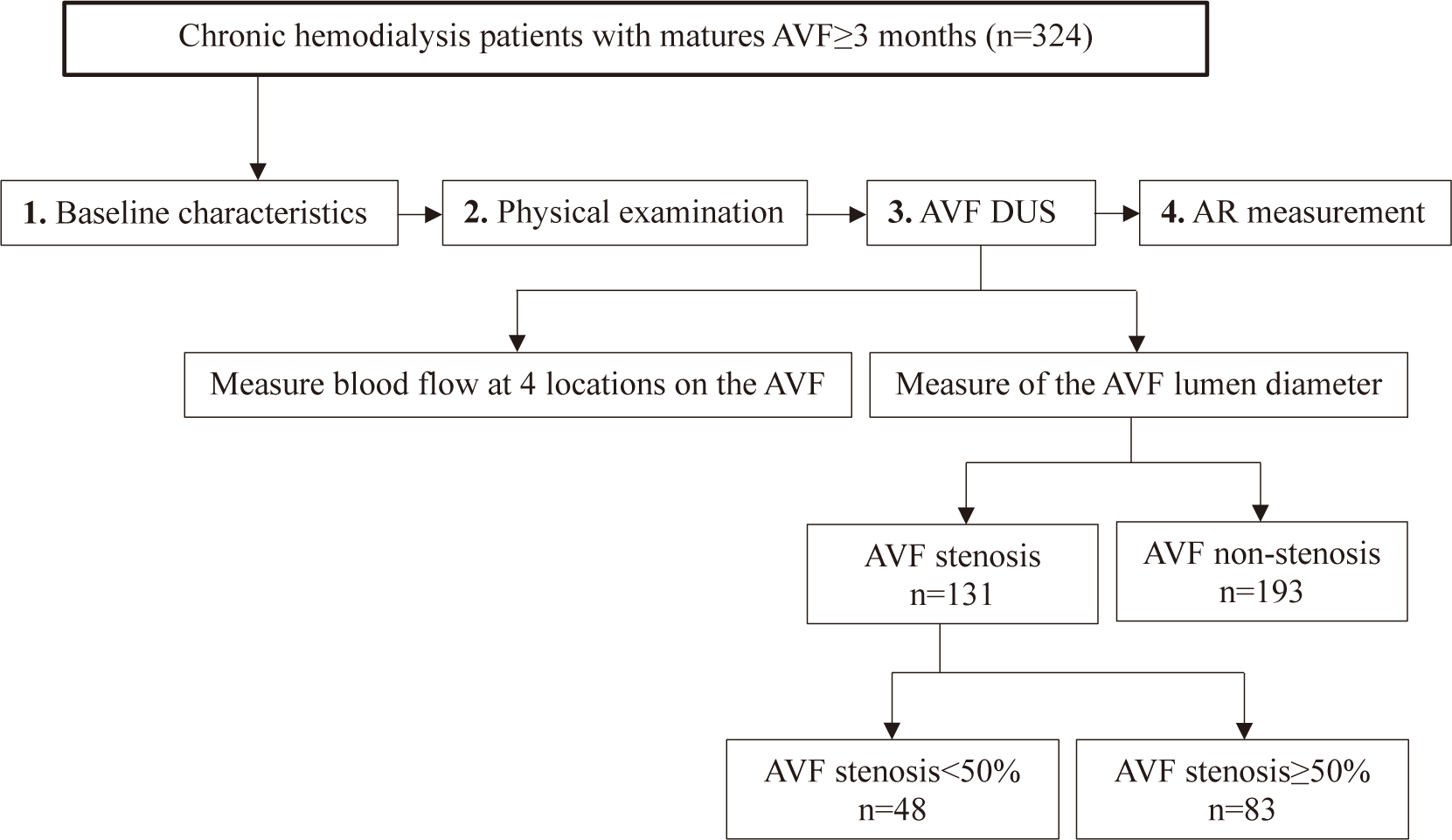
Data was analyzed using STATA16.0 (Stata, College Station, TX, USA). All categorical variables were described as frequency and percentage. All continuous variables were treated as if they were normally distributed because the sample size was greater than 30 [1]. All continuous data were analyzed. Differences in the characteristics of hemodialysis patients with non-stenosis, stenosis<50% and ≥50% were compared using Chi-squared test and ANOVA. The Bonferroni post-hoc test was used after ANOVA to identify which specific group differences (non-stenosis, stenosis<50%, and stenosis≥50%) are significant [17].Ten different cut-off points of AR (≥3% to 12%) were selected to calculate sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and areas under the curve (AUC) with their 95% confidence interval (CI) for distinguishing AVF stenosis as non-stenosis, stenosis<50% and stenosis≥50%. Statistical significance differences in the calculated AUC compared to an AUC value of 0.5 were assessed using receiver operating characteristic (ROC) curves. The optimal cut-off point was selected if its Youden’s J index was the highest. The Youden’s J index was calculated by adding sensitivity and specificity, then subtracting 100% [18].
3. RESULTS
The study recruited a total of 324 hemodialysis patients: 193 patients with AVF non-stenosis, 48 patients with AVF stenosis<50%, and 83 patients with AVF stenosis≥50%. Gender, age, BMI, the number of dialysis vintage years, hypertension, diabetes mellitus, history of previous CVC placement, history of previous AVF creation, current placed AVF, and left side of AVF were not signifcantly different across the three groups.
The mean AR of all patients was 7.14% (SD 8.89). The mean AR of patients with AVF stenosis≥50% was 10.45% (SD 14.03) which is higher than the mean AR of patients with AVF stenosis<50% (M 6.44, SD 6.19) and patients with non-stenosis (M 5.89, SD 5.76) (Bonferoni post-hoc test, p<0.01). There were seven cases with AVF thrombosis in the sample, of which six were patients with AVF stenosis≥50% and one was a patient with non-stenosis.
When examining the parameters derived from ultrasound blood flow measurements, it was evident that all access flow values (mL/min) at four different sites, Qmean, and Qmin were significantly lower in the patients with AVF stenosis≥50% compared to those with AVF stenosis<50% and those with non-stenosis (Bonferoni post-hoc test, p<0.05).
Patients who have symptoms such as collateral veins in the same arm, a positive arm elevation test, and a positive pulse augmentation test had a significantly higher percentage of AVF stenosis compared to those without these symptoms (p<0.05). These findings were presented in Table 1.
For detecting AVF stenosis<50%, the AR’s cutoff point of 3% provided the highest sensitivity (68.8%) whereas the AR’s cutoff point of 12% provided the highest specificity (88.4%). For detecting AVF stenosis≥50%, the AR’s cutoff point of 3% provided the highest sensitivity (88%) whereas the AR’s cutoff point of 12% provided the highest specificity (91.7%). No optimal cutoff point of AR is observed in the group of patients with AVF stenosis<50%. However, the optimal cutoff points of AR of 4% and 5% were observed in the group of patients with AVF stenosis≥50% with the highest Youden’s J index (data was not shown). For those patients with AVF stenosis≥50%, the AR cutoff point of ≥4% provided a sensitivity of 72.3% and a specificity of 46.9%. The AR cutoff point of 5% provided a sensitivity of 60.2% and a specificity of 58.9%. Both showed AUC of 0.6 (95%CI 0.54–0.65) (Table 2).
4. DISCUSSION
The study examines the validity of AR in screening AVF stenosis in hemodialysis patients against DUS. It became evident that AR did not offer value in the screening for AVF stenosis<50%. These results underscore the validity of using an AR cutoff value of ≥4% or ≥5% for the screening of AVF stenosis≥50%. These cutoff values were also consistent with the recommendations of KDOQI 2006 [19]. Our study also found that AR was highest in hemodialysis patients with AVF stenosis≥50% (10.45±14.03%) compared to those with non-stenosis and stenosis<50%. Several earlier studies have also reached the conclusion that AR is a valuable predictor of stenosis. For instance, Collins et al. conducted a study in 1992, demonstrating that AR serves as a prognostic indicator for venous stenosis. In cases where venous stenosis was corrected, there was a notable improvement in AR (rising from 21±3% to 36±3%, with p<0.05) [20]. A study conducted by Vega et al. showed that the mean AR was 9.5±6.6%, and this parameter proved valuable in diagnosing vascular access disorders, with an AUC of 0.84 (95%CI 0.73–0.95, p<0.01), demonstrating an association between AR and the risk of vascular access dysfunction [21]. While DUS has been assessed and proved valuable in various studies, it does come with a relatively elevated cost and dependence on the operator’s skills. Nonetheless, AR can serve as a cost-effective alternative to identify stenosis and predict access failure in hemodialysis patients. In conjunction with physical examination, AR offers a practical approach to monitor AVF stenosis in hemodialysis patients [22].
The demographic data of the three groups (non-stenosis, stenosis<50%, and stenosis≥50% on DUS) in the study showed no statistically significant difference (p-value>0.05). Dialysis vintage in years in the non-stenosis group lasted longer than in the<50% or ≥50% stenosis group at a level close to statistical significance (p=0.06). In assessing the utility of AR as a screening measure for AVF stenosis, our initial results involved examining the relationship between AR values and the presence or absence of AVF stenosis. The data in Table 1 indicates that the mean AR value in the research group (n=324) was 7.14±8.89%, AVF non-stenosis group (n=193) was 5.89±5.76%, AVF stenosis<50% group (n=48) was 6.44±6.19%, and AVF stenosis≥50% group (n=83) was 10.45±14.03%. When comparing between 3 groups: non-stenosis, stenosis<50% and stenosis≥50 AVF diameter on DUS, there was a statistically significant difference between the non-stenosis group and the stenosis group<50% (p-value<0.01), and there was also statistically significant between the non-stenosis group and the stenosis group<50% (p<0.05). Our study is similar to Raksasuk (2023) in that AR provided AUC of 0.82, sensitivity of 89% and specificity of 64% in screening for AVF stenosis≥50% whereas it provided AUC of 0.63, sensitivity of 73% and specificity of 80% in screening AVF stenosis<50% [23].
Six out of ten AR cutoff points displayed statistically significant differences, with decreasing pecentages observed in the order of AVF stenosis≥50%, AVF stenosis<50%, and AVF non-stenosis (Table 2). Numerous studies have similarly highlighted noticeable relationship between AR and stenosis. AR was a better predictor of AVF dysfunction (AVF stenosis) Vega (2018) [21]. A review aimed at evaluating AR among end stage hemodialysis patients concluded that high-grade venous stenosis is the most frequent underlying cause of AR. Therefore, AR may be used as a surveillance technique for the screening of AVF stenosis [24,25]. The presence of AR in hemodialysis patients can substantially compromise the effectiveness of dialysis, potentially diminishing the survival rates of these individuals. Consequently, it is imperative to regularly evaluate AR in hemodialysis facilities [26].
According to our results, AR did not have much significance for diagnosing AVF stenosis≥50% of diameter with an AUC of 0.6 at AR cut-off points of ≥4% or ≥5%. AR was inexpensive and can be performed routinely in hemodialysis centers, even in those centers with minimum equipment. Therefore, it should be used as an initial AVF screening tool before making the decision to use other methods. AR was measured every 2–3 months in patients receiving hemodialysis through an AVF [27]. In Vietnam, according to the Ministry of Health’s procedures on the frequency of testing values, BUNs are done every 1–3 months [28]. Three BUN samples are taken according to the procedure including peripheral, arterial and venous BUN. We used a two-needle method, which means three BUN concentrations were drawn from two available needle sites (artery and vein) at each hemodialysis shift and the BUN results (S, A, and V) were collected from the patient’s medical records. As the number of nephrologists and experienced dialysis doctors increases, physical examinations will be used as a combined tool to enhance the ability to diagnose AVF stenosis. Ultimately, the definitive diagnosis of AVF stenosis requires DUS or interventional angiography, procedures typically conducted at tertiary hospitals. AVF serves as the “heart” for end-stage chronic kidney disease patients undergoing maintenance hemodialysis. Early diagnosis and proper management of complications contribute to preserving and extending the life of the AVF. While AR may not excel in diagnosing AVF stenosis, its benefits for the AVF hemodialysis patient community are undeniable.
There were some limitations in our study. Firstly, we conducted the research in a single hemodialysis center with a relatively small number of participants with stenosis<50%, which restricts the generalizability of our findings to the broader hemodialysis population. Secondly, the gold standard for the diagnosis of AVF stenosis was digital subtraction angiography (DSA) but our study relied mainly on DUS. This study used DUS to ensure patient safe because DSA is invasive and can affects the patient’s residual kidney function.









