1. INTRODUCTION
Arteriovenous fistula (AVF) stenosis is one of the most common causes of malfunctioning vascular access in chronic hemodialysis patients [1]. Progressive stenosis gradually leads to a high risk of thrombosis and poor hemodialysis efficiency, which can contribute to lower survival rate among hemodialysis patients [2,3]. The National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) recommends that clinical examination is the fastest and most effective way to assess the AVF [3].
Duplex Doppler ultrasound (DUS) is commonly used as an alternative tool for screening and diagnosing AVF stenosis. DUS assis-tants in measuring the diameter and percentage of AVF stenosis, as well as measuring access flow thrombosis at various locations of the AVF. However, official guidelines recommending the measurement of access flow thrombosis, monitoring blood pressure (BP), or imaging to detect AVF stenosis, have not been well established.
Angiography, being costly and invasive, is not routinely used for screening AVF stenosis unless concurrent therapy is expected [4]. Non-invasive methods such as physical examinations (PEs) and hemodynamic parameters (measuring blood flow, determining BP, and measuring blood recirculation) are valuable alternatives for AVF patency [5–8]. In this study, DUS was employed as the standard method for diagnosing AVF stenosis in chronic hemodialysis patients with a dialysis vintage, and was compared against PEs and he-modynamic parameters, including access flow AVF at four different sites (brachial artery, anastomosis, 5 cm from the anastomosis and 10 cm from the anastomosis).
2. MATERIALS AND METHODS
A cross-sectional study was conducted at the Department of Hemodialysis, Cho Ray Hospital from September 2020 to September 2022. The study followed the Standards for Reporting of Diagnostic Accuracy (STARD) statement for reporting diagnostic accuracy studies [9].
The inclusion criteria included chronic hemodialysis patients aged 18 years and older, who had undergone dialysis vintage through AVF for at least 3 months and consented to participate. A systematic review suggested that AVF maturation typically occurs around a the median of 3.49 months [10]. Complications and stenosis were reported at 1.43 thrombotic events per 1,000 patient days at 3 months after AVF creation [10], which explains why the study recruited patients with AVF installed for at least three months.
Patients who had hemodialysis with arteriovenous graft or catheterization were excluded from the study. One researcher consecutive-ly approached and screened all patients for their eligibility. Eligible patients were provided with an explanation of the purpose of the study and given consent forms. If they agreed to participate, they were asked to sign the consent forms before data collection com-menced.
As the diagnosis accuracy of AVF stenosis was prioritized in this study, the sample size was calculated based on the formula to es-timate a specificity. Previous studies reported specificities ranging from 70% to 85%. Thus, we used the specificity of 70% with a 95% confidence interval with of 10% for our calculation to achieve the higher sample size. Moreover, as this study was the first of its kind in Vietnam, the prevalence of AVF stenosis was unknown. Based on our calculation with different assumptions of prevalence ranging from 0.3 to 0.5, the prevalence of 0.5 resulted in a higher sample size. Therefore, a sample size of 324 hemodialysis patients was needed for our study.
Patients meeting the above inclusion criteria were examined before hemodialysis, and parameters were collected. Each patients un-derwent AVF DUS outside of their hemodialysis sessions and had tested five urea samples taken, including: two samples (before and after hemodialysis) to calculate spKt/V, and three samples (Blood Urea Nitrogen in the peripheral vein, arterial line, and venous line) to calculate access recirculation (AR). This protocol was repeated across different hemodialysis shifts until the required number of pa-tients was reached.
Age, gender, body mass index (BMI), the number of dialysis vintage years, hypertension, diabetes mellitus, history of previous cen-tral venous catheter (CVC) placements, and history of previous AVF creation were collected through patient interview using a ques-tionnaire. Information regarding swollen fistula arm, collateral veins in ipsilateral arm, positive arm elevation test, positive pulse aug-mentation test, and the percentage of AVF stenosis, AVF thrombosis access flow (mL/min) at different sites (Brachial artery [Qa], Anas-tomosis, 5 cm from the anastomosis, 10 cm from the anastomosis) were obtained from medical records. The percentage of AVF steno-sis was classified as non-stenosis and stenosis ≥50%. All chronic hemodialysis patients underwent DUS, a PE, and measurement of hemodynamic parameters during hemodialysis.
DUS was conducted by a qualified expert in cardiovascular ultrasonography using 7.5 MHz probe and Winno E10 color flow du-plex machine. The DUS result included both blood flow and luminal diameter measurements along the vascular access, from the bra-chial artery to the subclavian vein of AVF arm. The percentage of AVF stenosis was classified as non-stenosis and stenosis ≥50% [11,12].
Access blood flow (Q) was calculated using the formula, Q=Cross-sectional area (cm2)×minimal velocity (cm/s)×60 where cross-sectional area (cm2)=π d2/4 (d: diameter) [13,14]. Access blood flow (Q) was recorded at four different locations along the AVF, includ-ing the brachial artery feeding the AVF (Qa), anastomosis, 5 cm and 10 cm from the anastomosis. Qmin was defined as the minimal blood flow value among these four sites. Luminal diameter reduction of ≥50% was used as criterion to confirm AVF stenosis for the optimal sensitivity [12].
All guidelines stipulate that examination and diagnosis of AVF stenosis should be conducted by a qualified nephrologist [15,16]. The PE included inspection of the AVF arm for swelling and evidence of collateral veins in related areas of vasculature in the fistula arm, chest and neck. The entire AVF tract examination, with and without arm elevation and pulse augmentation tests, was evaluated for outflow and inflow.
The arm elevation test was scored as positive when the AVF failed to collapse while the arm was elevated above the heart. The pulse augmentation test involved completely occluding the AVF several centimeters downstream from the arterial anastomosis with one hand, while the other hand was used to assess the quality of the pulse. This test was deemed positive when augmentation test was positive when the pulse failed to augment while the vein was occluded.
The hemodynamic parameters were collected during hemodialysis within a week of DUS evaluation. These parameters included AR, single-pool Urea Kt/V, venous access pressure ratio (VAPR), blood flow rate (Qb), venous pressure (Pv), artery pressure (Pa), intra-access pressure vein (PiaV), intra-access pressure artery (PiaA) [5].
AR was evaluated by the two- needle urea-based method. The percent recirculation was calculated using the
formula: [17] where “S” represents the
concentration of Blood Urea Nitrogen (BUN) in the peripheral vein, “A” represents the concentration in the arterial line, and “V” repre-sents the concentration in the venous line during hemodynamic.
The blood samples were drawn after slowing the blood flow or stopping dialysate flow at the end of dialysis. It is crucial to ensure the needles are in the appropriate position and the lines are not reversed before blood withdrawal for BUN measurements. The single-pool urea Kt/V (spKt/V) was calculated by formula: spKt/V=–ln(R–0.008xt)+(4–3.5xR)×0.55×UF/V. In which, Kt/V=2.2–[3.3×{R–(0.03–UF/W)}], R=post dialysis BUN from venous line; pre-dialysis BUN from arterial line; UF: ultrafiltration (kg); W: body weight post dialysis (kg); K: urea clearance of dialyzer (L/h); t: dialysis time (h); V: urea distribution volume (L) [18].
Blood flow rate (Qb) was the rate of blood pumped during hemodynamic measurements. Venous (Pv) and arterial access pressure (Pa) referred to the pressure needed to infuse blood through the venous needle and withdraw blood through the arterial needle, respec-tively. VAPR was defined as the ratio between venous access pressure (Pv) and mean BP. The average BP is calculated as BP (mmHg)=diastolic BP+1/3×(systolic BP–diastolic BP). PiaV and PiaA was the intravascular pressure at the venous and the arterial needle site, respectively.
Data was analyzed using STATA16.0 (Stata Corporation, College Station, TX, USA). Continuous variables underwent evaluation through the Kolmogorov-Smirnov test, with a p-value>0.05 indicating normal distribution. Age (in years), BMI (kg/m2), the number of years on dialysis (dialysis vintage), and access flow (in mL/min) at four different locations were described using the median and inter-quartile range (IQR) due to their non-normal distribution. Categorical variables, including gender, hypertension, diabetes, a history of previous CVC placements, a history of previous AVF creation, swollen fistula arm, collateral veins in ipsilateral arm, positive arm elevation test, positive pulse augmentation test, AVF stenosis (non-stenosis, stenosis ≥50%) were described using frequency and per-centage. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of PE and hemodynamic parameters were calculated based on Duplex DUS as the standard in detecting AVF stenosis. Additionally, the Area Under the Curve (AUC) and its 95% Confidence Interval (95%CI) were calculated. An AUC >0.7 was considered clinically significant [19].
3. RESULTS
The median age of 324 chronic dialysis patients was 49 years (IQR 38–61), ranging from 38 to 61. Women counted 53.7% of the sample. The BMI was 20.85 (IQR 18.38–23.91), ranging from 18.38 to 23.91. The median number of dialysis vintage years was 8 (IQR 5–13). Hypertension was presented in 82.7%, while diabetes was present in 15.4%. The percentage of patients with a history of previous CVC placements was 49.7%. The majority of patients (80.25%) had a history of previous AVF creation. Swollen fistula arm and collateral veins in ipsilateral arm were observed in 9.9% and 8.0% of the sample, respectively. Positive result were found 50.9% of patients with a positive arm elevation test and 38% with a positive pulse augmentation test. DUS detected 131 (40.4%) patients having some percentages of stenosis, of which 83 (63.4%) patients having ≥50% of stenosis. The percentage of patients having AVF throm-bosis was 2.2%. The access flow was as follows: 1,216 mL/min (IQR 955.75–1,730.5) in brachial artery (Qa), 1,168 mL/min (IQR 816–1,934.75) in anastomosis, 998.5 mL/min (IQR 685–1,810) in 5 cm from the anastomosis, and 825 mL/min (IQR 522.75–1,570.5) in 10 cm from the anastomosis. Data was detailed in Table 1.
There was no significant differences in age, gender, BMI and medical history were not different between the stenosis and non-stenosis groups on DUS (p>0.05). However, the dialysis vintage (years) of the non-stenosis group was longer than that of the stenosis group (p=0.02). Diagnostic tests for AVF stenosis (collateral veins in ipsilateral arm, positive arm elevation test and positive pulse aug-mentation test) accounted for a higher proportion in the stenosis group compared to that of the non-stenosis group (p<0.01). Further-more, measurements of the access flow of four AVF sites (brachial artery, anastomosis, 5 cm from the anastomosis and 10 cm from the anastomosis), were all decreased in the stenosis group (p<0.01). Data was detailed in Table 2.
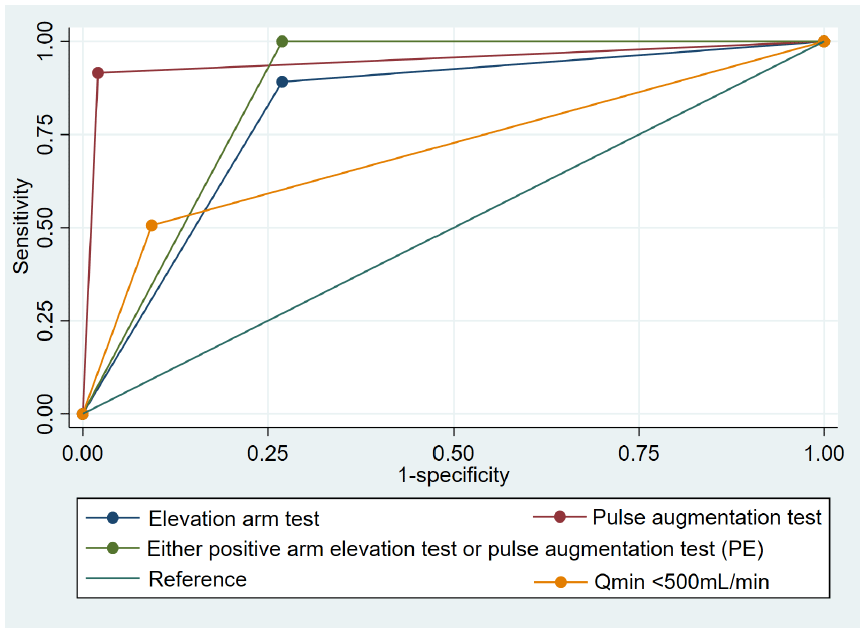
Table 3 shows sensitivity, specificity, PPV, NPV, and areas under the curve of different non-invasive tests compared to DUS. The pulse augmentation test exhibited the highest AUC (0.96; 95%CI 0.92–1.00), and the highest sensitivity and specificity (91.57% and 97.93%, respectively). Following this, the arm elevation test demonstrated the second highest AUC (0.74, 95%CI: 0.67–0.81), with high sensitivity (89.16%) but moderate specificity (73.06%). The Qmin<500 mL/min of DUS ranked third for the AUC (0.71, 95%CI: 0.63–0.78), showing high specificity (90.67%) but low sensitivity (50.60%). In contrast, the Qa<500 mL/min at brachial feeding artery showed low sensitivity (9.64%), but high specificity (98.96%), resulting in an AUC of Qa 0.54 (95%CI: 0.47–0.62). Combining the arm elevation test and pulse augmentation test provided an AUC of 0.87 (95%CI: 0.82–0.91) with a sensitivity of 100% and a speci-ficity of 73.06%.
AUC, area under the curve; Qa, blood flow at brachial artery feeding AVF; Qmin, the minimal blood flow value of the 4 sites (brachial artery feeding the AVF, anastomosis, 5 cm and 10 cm from the anastomosis); VAPR, venous access pressure ratio; Sens, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value.
Fig. 1 illustrates the receiver operating characteristics curves of positive pulse augmentation test, either positive arm elevation test, positive arm elevation test, and the Qmin<500 mL/min that provide AUC higher than 0.7.
4. DISCUSSION
This study evaluated the precision of PE and hemodynamic tests in comparison to DUS, revealing that arm elevation and pulse augmentation tests exhibited the highest accuracy in indentifying AVF stenosis compared to other non-invasive tests. Consistent with the guidelines from the NKF-KDOQI [3] and the European Society of Vascular Access [20], which recommend regular PE by a knowl-edgeable and experienced health practitioner on a monthly bases for surveillance and early detection of AVF dysfunction [3]. Our findings emphasized the importance of these clinical assessments in clinical practice.
The PE included systematic assessment involving inspection, palpation and auscultation of the AVF and related regions. During the arm elevation test, the patient’s arm was raised, and if blood failed to promptly fill the AVF due to gravitational effects, the AVF would gradually collapse. Typically, the site of stenosis or occlusion is indicated by the junction between collapsed and non-collapsed sections of the AVF. Clinical examinations have demonstrated that both pulse augmentation and arm elevation tests exhibit high sensitivity in detecting AVF stenosis [21]. Moreover, the pulse augmentation test is particularly sensitive to stenosis occurring in the artery feeding, anastomosis, and juxta anastomosis of AVF. Any issues with the arterial system from the anastomosis downstream can impact the extent to which the pulse is augmented. However, this pulse augmentation may not be noticeable if severe stenosis is pre-sent of the AVF.
The presence of either test provided perfect sensitivity of 100%, but low specificity of 73.06% to detect AVF stenosis. Chen et al. [22] developed a pulse- and thrill-based scoring system for diagnosing AVF stenosis. According to this scale, more than 75% were diagnosed with AVF stenosis, with a sensitivity of 80.39%, a specificity of 78.79%, a PPV of 85.42% and a NPV value of 72.22%. In Mishler et al. [23] study of 59 AVF stenosis cases diagnosed by angiography, they performed PE in 59 consecutive patients, achieving an accu-racy rate of 91% in predicting stenosis. Our study supports the recommendations of NKF-KDOQI [3] and the European Society of Vas-cular Access [20] that PE was the crucial tool for AVF surveillance and diagnosis, given its ease of learning, performance, speed, and cost-effectiveness.
DUS was selected to confirm AVF stenosis, although it was less accurate than angiography. A study involving 35 AVF access showed that DUS significantly diagnosed stenosis, which was confirmed by angiogram [15]. However, angiography, when used solely for diagnostic purposes without concomitant treatment, should be avoided [4]. The variability in dilatation levels at different points in the AVF access challenges the use of luminal diameter to define the AVF reduction and represents a weakness of DUS compared to angiography [2]. To address the subjectivity in evaluation, reduce costs, and minimize the risks associated with contrast use in angi-ography, we utilized the DUS mapping (approximately 20 USD in Vietnam). This approach involves a comprehensive mapping of the entire AVF and blood flow measurement at 4 sites rather than solely at brachial feeding site (Qa).
In our study, seven cases were found to have thrombosis in the AVF. Arteriosclerosis and intimal hyperplasia might be the most common causes of AVF stenosis. Raju used the ratio of the peak systolic velocity (PSV) between the suspected area of stenosis and the pre-stenosis, and found that DUS had the best sensitivity (95.5%), but moderate specificity (57.1%) [15]. Despite this, due to its the cost effectiveness, comparable results and adding other readily available information such as access blood flow, PSV, thrombosis, DUS was remains recommended for disanosing and servuilling AVF stenosis [2,3,20].
In addition to diameter measurements, DUS could provide valuable information on the blood flow volume using dilution tech-niques during hemodialysis or at any time [6,13]. For the native AVF, blood flow through the brachial artery feeding the AVF (Qa) is considered a functional marker [13]. In a healthy AVF, Qa typically ranges between 500–1,500 mL/min. Values below this range are associated with an increasing risk of inadequate hemodialysis and access thrombosis, while higher values may indicate conditions like heart failure or steal syndrome. According to NKF-KDOQI guidelines [3], an AVF should be referred for fistulography if Qa<400–500 mL/min. Studies have reported a wide range of Qa from 300 mL/min to 900 mL/min for detecting AVF stenosis [2,24,25]. In our study, we found that measuring blood flow at any of 4 sites (or Qmin)<500 mL/min was more sensitive than Qa alone in AVF stenosis diagno-sis. Measuring blood flow at 2 to 4 locations along the graft, particularly in areas without luminal narrowing or turbulent blood flow as detected by color Doppler, has been used to improve the accuracy of arteriovenous graft evaluation [8].
A variety of hemodynamic parameters were selected in this study to measure changes in functional hemodynamic endpoints. The findings showed that most of the hemodynamic parameters had moderate AUC values, except two sample urea-based AR ratio. AR refers to a situation that can occur during hemodialysis that involves using a dialyzer to remove waste products and excess fluid from the blood. AR reduces the urea concentration in the blood entering the dialyzer to at least 10% [17]. When stenosis occurs anywhere along the AVF, it can increases the AR ratio by ≥5%. However, in this study, the AR ratio ≥5% showed a low AUC of 0.60 (95%CI: 0.53–0.68).
AVF stenosis can lead to changes in regional hemodynamics, resulting in reduced access flow volume, followed by increased recir-culation, decreased of Kt/V ratio, high venous pressure, reduced thrill, and prolonged post-dialytic bleeding as well as AVF thrombosis. As a result, the presence of AR may lead to under dialyzed patients and contribute to increase morbidity and mortality in chronic he-modialysis patients [26].
According to the European Best Practice Guidelines Expert Group (EBPG) guidelines [4], the decision on whether clinical examina-tion alone is sufficient to confirm stenosis or an imaging examination is necessary depends mainly on local customs and practice. Our study suggests that if a patient tests positive for pulse augmentation test, arm elevation test, or both, they should be referred to DUS to detect AVF stenosis. Any reduction of luminal diameter ≥50% compared to the adjacent vessel on the inflow side, and any access site with blood flow<500 mL/min, indicates a high likelihood of AVF stenosis.
Despite the intensive research, this study had several limitations. It was conducted at Cho Ray hospital, a tertiary care facility serv-ing patients of varying disease severity from Ho Chi Minh City and neighboring regions, which may limit the generalizability to other settings. Additionally, angiography is the gold standard for detecting AVF stenosis, but this study used DUS due to its non-invasive nature, lower cost, and routine preference in clinical practice. Although DUS is an accurate alternative, it may not provid the sae level of detail as angiography. However, it is worth noting that Cho Ray is the first to implement DUS according to the high standards of the International Society Nephrology.
5. CONCLUSION
Despite the limitations, this study demonstrates that PEs including arm elevation test and pulse augmentation test exhibit high sensi-tivity and specificity for early detection of AVF stenosis and should be considered as initial screening methods in clinical practice. Patients with positive arm elevation test and pulse augmentation test should be prioritized for DUS.









