1. INTRODUCTION
Community-acquired pneumonia (CAP) is the leading cause of death among many infectious diseases, with more than 1.6 million deaths worldwide annually, of which 0.7-1 million cases of children under five years of age [1, 2]. From a report to the United Nations Children’s Fund (UNICEF) and World Health Organization (WHO) in 2004, the Ministry of Health of Vietnam estimated about 4500 children under five years of age died due to CAP annually [3]. The most common causative agent of CAP was Streptococcus pneumoniae, which is usually found in the nasopharyngeal carriages and causes the highest mortality rate among pathogens [4-6]. Empirical therapy for nonsevere pneumonia in children was recommended for an antimicrobial regimen including amoxicillin or ampicillin to provide good coverage of S. pneumoniae [7].
In recent years, epidemiological surveys showed that antibiotic resistance had been a global problem, becoming one of the urgent health challenges for the next decades [8]. The resistance rate of S. pneumoniae to macrolides and many other antibiotics has considerably increased, especially in Asian countries [9, 10]. In Vietnam, the proportion of erythromycin-resistant S. pneumoniae was 92.2%, the highest among 11 Asian countries [9]. In 2011, also in Vietnam, pneumococcal isolates from respiratory infections developed resistance to broad-spectrum antibiotics such as cotrimoxazole (91%), tetracycline (78.6%), chloramphenicol (67.9%), and second-generation cephalosporins such as cefuroxime (71.4%), cefaclor (87.6%) [11].
Due to the overuse of antibiotics and the rapid increase in antimicrobial resistance, it is necessary to revise the guidelines regularly to gain clinical effectiveness and lower the clinical failures, mortality rate, and the burden of treatment costs on the patients [12, 13]. In addition, because the resistance rate could vary according to healthcare settings, it should be investigated and continuously updated.
In line with the national action plan on antimicrobial resistance, we investigated the antimicrobial susceptibility of S. pneumoniae isolates collected from CAP children at three hospitals in Quang Nam province and Da Nang city from September 2017 to September 2018. This study aims to provide clinicians with critical and updated data on antibiotic resistance patterns and support them to select appropriately and safely antibiotics for empirical treatment of CAP.
2. MATERIALS AND METHODS
This cross-sectional and in-vitro study was conducted in the middle region of Vietnam at the pediatric departments of three hospitals, including Da Nang Hospital for Women and Children (DN) - a secondary referral hospital in central Vietnam; Quang Nam Hospital for Women and Children (QN1) - the representative hospital in the urban area of Quang Nam province; and General Hospital at Northern Mountain of Quang Nam (QN2) - the representative hospital in the rural area of Quang Nam province from September 2017 to September 2018. The number of beds of DN, QN1, and QN2 is about 500, 370, and 350, respectively. In QN1 and QN2, the beds can be added depending on the number of patients admitted to the hospital. Annually, about 4000 children are admitted to Da Nang Hospital for Women and Children due to acute respiratory infections, whereas this figure for Quang Nam Hospital for Women and Children and General Hospital at Northern Mountain of Quang Nam is approximately 3500.
Where:
-
- n: Minimum sample size
-
- Z 1-α/2 : The number of standard errors away from the mean (Z 1-α/2 = 1.96 for a level of confidence of 95% or a level of significance of 5%)
-
- P: The estimated proportion of the population p = 0.3, based on previous research [15-17] (The pneumococcal nasopharyngeal carriage rate in children with CAP was 21.5%, 46%, 28.6%, respectively)
-
- d: The distance, in either direction, from the population proportion (d = 0.05)
The minimum sample size was calculated at 323 children and rounded to 360. The number of patents of each hospital was 120. The association between qualitative variables was analyzed with Pearson’s chi-square test using SPSS statistics software 20.0.
Clinical specimens were collected by a convenient sampling method with the support of hospitals. Nasopharyngeal aspirates (NPA) of CAP inpatients from 2 months to 60 months who had not received antibiotics for more than 24 hours before hospitalization were obtained by nurses of the hospitals. The collection was performed based on the guidelines of the Vietnam Ministry of Health [18] and the Center for Disease Control and Prevention [19].
The samples were stored in sterile vials and labeled with the collection date, name, age, gender, and ID number of the patients, then transported to the hospital’s laboratory for culture and isolation.
The specimen was cultured on a Blood Agar (BA) plate with 5% sheep blood and then incubated at 35-37°C in 5% CO2 for 16-24 hours. The identification of pneumococcal isolates underwent steps such as observation of colony morphology, gram staining, optochin susceptibility testing, bile salt solubility [20]. The suspected strains were confirmed by Buker’s MALDI-TOF (matrix-assisted laser desorption/ionization - time-of-flight) mass spectrometry. S. pneumoniae strains were kept in Brain Heart Infusion (BHI) broth with a supplement of 5% sheep blood (manufactured by Nam Khoa. LTD) and 20% glycerin and stored at -70°C.
The antimicrobial susceptibility testing (AST) of S. pneumoniae isolates were performed with 12 antimicrobials clinically used in the three hospitals by the disk diffusion method following guidelines established by the Clinical and Laboratory Standards Institute (CLSI) and, when required, by minimum inhibitory concentrations (MICs) by E-test [21]. Kirby-Bauer disk diffusion method was used for testing of erythromycin (E:15 μg disc), azithromycin (AZM:15 μg disc), clindamycin (DA: 2.0 μg disc), levofloxacin (LEV: 5.0 μg disc), trimethoprim + sulfamethoxazole (SXT: 25 μg disc), vancomycin (VA: 30 μg disc), linezolid (LZD: 30 μg disc) (Oxoid). For the other antimicrobials such as penicillin G (PG), amoxicillin (AC), amoxicillin + acid clavulanic (XL), cefotaxime (CT), ceftriaxone (TX), the MICs were identified by using E-test strips (BioMérieux).
The inoculum was prepared by direct colony suspension method using colonies from overnight-cultured BA plates. The turbidity of suspension was adjusted to 0.5 McFarland, and suspension should be used within 15 minutes after preparation. The suspension was streaked evenly over the surface of Mueller-Hinton blood agar (MHBA) plates using a sterile cotton swab. The plate cover was slightly opened for 5 minutes to absorb excess liquid into the agar fully. The antibiotic disc or E-Test strips was applied to the surface of the MHBA within 15 minutes of inoculation and then gently pressed to ensure that it was entirely in contact with the agar. The MHBA plates were incubated at 35-37°C in 5% CO2 for 18-24h [22-24]. Quality control of antibiotic discs and E test strips was performed using strain S. pneumoniae ATCC 49619 [21, 25].
After 18-24 hours of incubation, results were read by the reflected light method. The inhibition zones of the antibiotic discs were measured using a ruler or calipers. The MIC was identified at the point where the ellipse intersects the scale, and the growth lawn was distinct. If the intersection point fell between two-fold dilutions, the MIC value was rounded up to the highest value. If the intersection differs on either side of the strip, MIC was identified as the greater value [23]. MIC breakpoints or inhibition zones for defining interpretive susceptibility were published by the Clinical and Laboratory Standards Institute (CLSI) [21].
Multi-drug resistance (MDR) was defined as acquired resistance to at least one agent in three or more antimicrobial groups [26-29].
Data were processed by using excel version 16.49 and analyzed by using SPSS statistics software 20.0. Variables specific to the research population used such as hospitals (DN, QN1 and QN2); age groups (2-23 months and 24-60 months); gender (Male, female); culture (positive, negative); isolation (positive, negative). The association between qualitative variables was analyzed using Pearson’s chi-square test with a level of confidence of 95%.
The study was approved by the Danang Hospital for Women and Children, two Quang Nam Provincial Hospitals, and the Department of Health of Quang Nam Province (approval decision No 3287/QÐ-UBND on September 08, 2017). Accordingly, the hospitals supported our research on clinical specimens and the isolation and identification of pneumococcus.
3. RESULTS
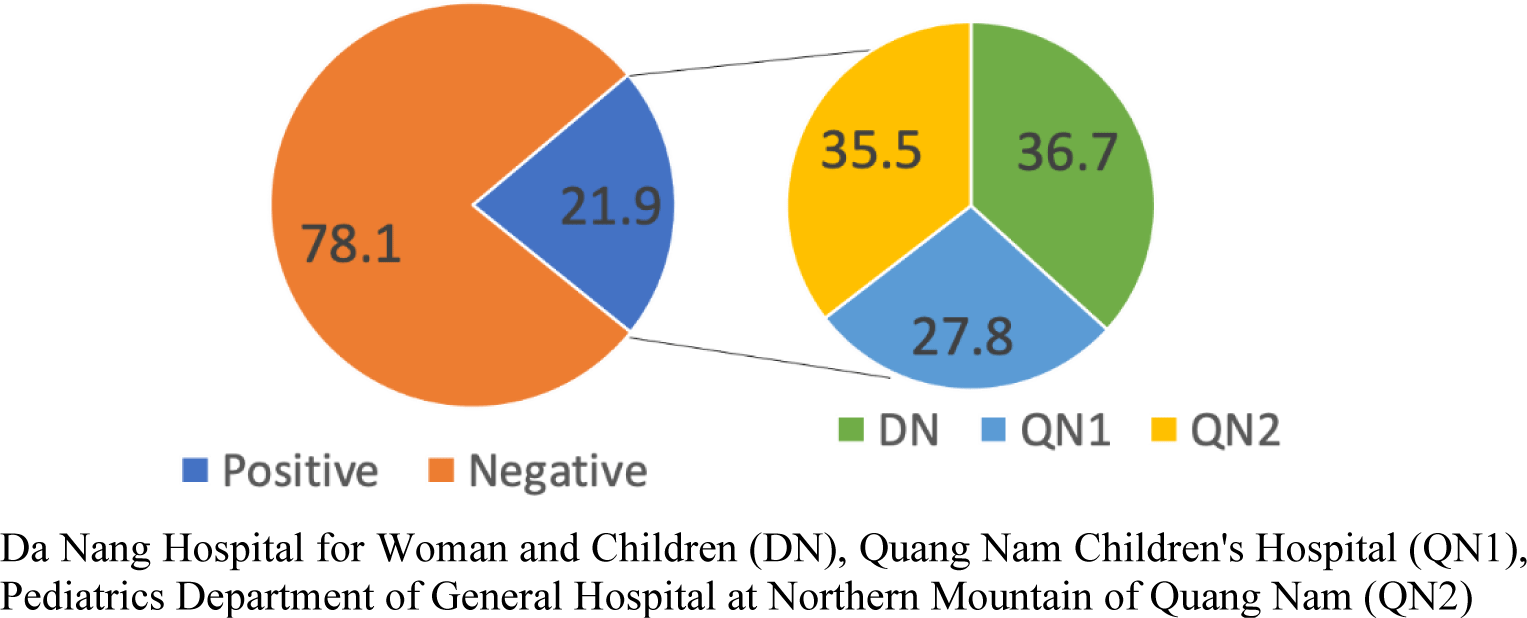
Overall, among 360 children with CAP in our study, there was a significantly higher prevalence of CAP in males than females (61.1% vs. 38.9%, p < 0.001). The majority of CAP children (62.2%, 224/360) aged between 2 – 23 months, while 37.8% (136/360) of cases aged from 24 to 60 months with significant difference (p < 0.001). In general, the prevalence of pneumococcal pneumonia (culture-confirmed with S. pneumoniae) was 21.9% (79/360 CAP cases). There was no significant difference among the three hospitals with the respective prevalence of pneumococcal pneumonia in each hospital were 24.2%, 18.3%, and 23.3% in DN, QN1, and QN2 (p = 0.498) (Table 1). As of 79 S. pneumoniae isolates, strains collected at QN2 and DN took up higher proportions (36.7% and 35.55, respectively) of the total pneumococcal isolates, while QN1 occupied a lower proportion, 27.8% (Figure 1). There was no significant difference in the S. pneumoniae-infected rate between the three hospitals (p = 0.498), between 2 age groups (p = 0.571), and also between two genders (p = 0.217).
A total number of 79 S. pneumoniae strains was initially isolated from 360 CAP cases. However, only 56 S. pneumoniae strains were tested for antimicrobial susceptibility. The missing was due to various reasons, including cell death (caused by freezer malfunction, autolysis during the transportation from Da Nang to OUCRU lab in Ho Chi Minh), strains lost, or contamination.
Regarding the AST results, a hundred percent of S. pneumoniae isolates (56 isolates) were resistant to macrolides, including erythromycin and azithromycin. Also, the high prevalence of pneumococci isolates was resistant to clindamycin and sulfamethoxazole-trimethoprim at 96.4 and 87.5%, respectively. Conversely, CAP S. pneumoniae isolates showed a very low resistance rate to β-lactams including penicillin G (3.6%), amoxicillin-clavulanate (1.8%), amoxicillin (1.8%), cefotaxime (1.8%), and ceftriaxone (3.6%). Especially, all S. pneumoniae in the study were susceptible to levofloxacin, vancomycin, and linezolid (Figure 2).
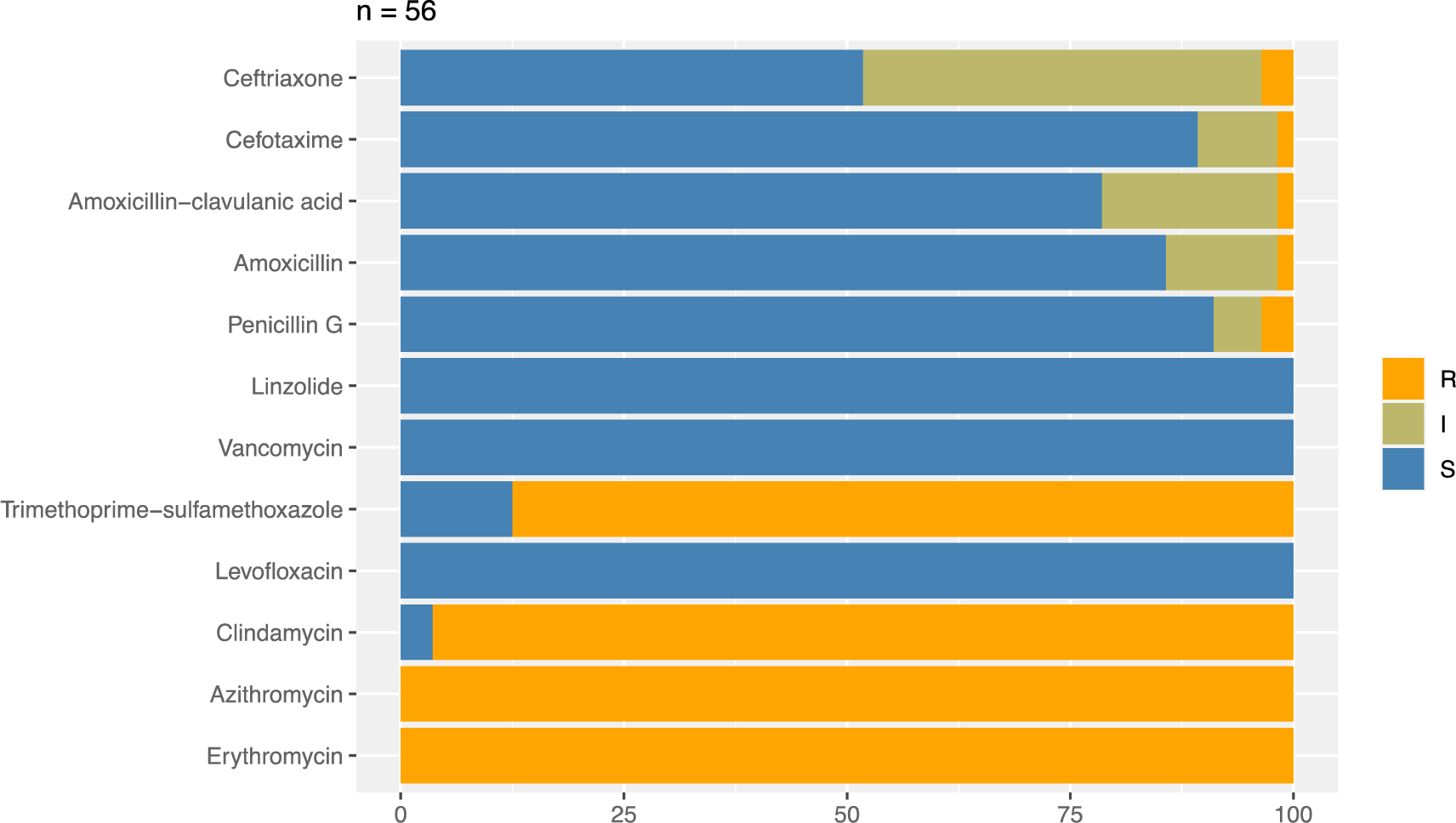
MICs of penicillin G, amoxicillin-clavulanate, amoxicillin, cefotaxime, and ceftriaxone for 56 tested pneumococcal isolates were identified by the gradient method using E- test and demonstrated in table 2.
The MICs of penicillin G and amoxicillin for S. pneumoniae isolates ranged from 0.016 to 12 μg/mL, in which 91.1% of S. pneumoniae isolates with penicillin MIC ≤ 2.0 μg/mL and 92.9% with amoxicillin MIC ≤ 3.0 μg/mL.
For amoxicillin + acid clavulanic (co-amoxiclav), the range of MICs on 56 S. pneumoniae isolates was between 0.016 and 16 μg/mL. The MICs below three μg/mL were observed in 93% of the total isolates.
For the remaining two antimicrobials, the result found that 89.3% of pneumococcal isolates exhibited cefotaxime MIC ≤ 1.0 μg/mL while the rate of strains with ceftriaxone MIC ≤ 2.0 μg/mL was 96.4%.
Overall, the proportion of MDR S. pneumoniae was 83.9% (47/56 isolates). In particular, the proportion of MDR S. pneumoniae at each hospital, including DN, QN1, and QN2, was 77.3% (17/22 isolates), 86.7% (13/15 isolates), and 89.5% (17/19 isolates), respectively. There was no significant difference between these three hospitals in the rate of CAP caused by MDR S. pneumoniae (p = 0.538).
4. DISCUSSION
In diagnoses of lower respiratory tract infections, NPA specimens were commonly used in many studies with high specificity [30-33] and less invasive in children [34]. Regarding the recovery rate, NPA indicated the highest rate of isolates of S. pneumoniae (33%) while oral pharyngeal swab (OPS) had the lowest rate with 20% [30]. The rate of detection of S. pneumoniae in NPA specimens collected from patients with CAP in our study was 21.9%. This proportion was similar to the result of the survey of Negash (21.5%) [15] and much higher than that of a study carried out on children aged from 0 to 12 months with CAP in China (13.9%) [33] and South Korea (2%) [32].
The discrepancy between pneumococcal carriage rates of patients may be influenced by the patient’s exposure to antibiotics before sample collection. It is difficult to determine the exact recall history and the detection of antibiotics in urine samples [15]. Children who had been treated with antibiotics before swabbing had significantly lower rates of S. pneumoniae than patients who had not received prior antibiotics [35]. Besides, techniques used to detect the presence of pneumococci in specimens also affected the results. For example, culture methods might show lower positive cases than PCR (polymerase chain reaction) [36].
Our results in figure 2 have demonstrated that all isolates of S. pneumoniae are entirely resistant to macrolides, including erythromycin and azithromycin. The trend of macrolide resistance is increasing compared to previous years in Vietnam. In 2004 and 2012, the rate of drug resistance of S. pneumoniae to erythromycin was 92.1% and over 99%, respectively [9, 11]. The prevalence of macrolide-resistant pneumococcal isolates in Vietnam is relatively similar to other Asian countries [31-33]. Such a high resistance rate could be attributed to the majority of resistance genes among pneumococcal strains in Asian countries [37], especially the erm(B) gene in 97.9% of macrolide-resistant strains of S. pneumoniae [10]. This gene encodes ribosomal methylase that can dimethylate the subunit of 23S rRNA, which is the main target site of macrolides [38]. This resistance mechanism also enables S. pneumoniae to develop resistance to other antibiotics, such as lincosamides. This may be an explanation for the high rate of isolates resistant to clindamycin in our study (96.4%) and the previous survey in Vietnam (85%) [11].
In the face of the high level of antimicrobial resistance, whether macrolides or clindamycin are still clinically effective for treating CAP caused by S. pneumoniae remains a controversial question. There may be some differences between in vitro and in vivo results due to the influence of host-related factors such as the immune system, the nature and severity of the infection, and the type and dose of the infection. Numerous reports state that macrolides enhance the efficacy of combination therapy, especially with β-lactam antibiotics [39-41]. Some authors suggest that the clinical benefit of macrolides does not come from their antibacterial properties but rather their anti-inflammatory effects. The anti-inflammatory effects of macrolides are illuminated by their ability to inhibit the production of interleukin-8 of bronchial epithelial cells [42]. In addition, macrolides can inhibit protein synthesis and reduce virulence factors [43, 44]. Therefore, adding macrolides may provide a better antibacterial spectrum, especially atypical agents.
On the other hand, our study found that 100% of S. pneumoniae isolates were susceptible to vancomycin, linezolid, and levofloxacin. This result is accordant with surveys carried out previously in Vietnam and Asian countries [11, 45, 46]. Therefore, using vancomycin in treating respiratory infections caused by multi-drug resistant pneumococcal isolates would undoubtedly produce good outcomes. However, it should be noticed that there was the emergence of vancomycin-tolerant S. pneumoniae isolates in some countries [47, 48]. Therefore, vancomycin should be prescribed as an alternative therapy in the cases caused by penicillin-resistant pneumococcal isolates (MIC ≥ 4.0 μg/mL) [49].
Regarding linezolid and levofloxacin, a study on pneumococcal isolates collected worldwide illustrated that 100% of S. pneumoniae strains were susceptible to these antibiotics [50]. In the guidelines of IDSA (Infectious Diseases Society of America), linezolid and levofloxacin are considered to use as alternatives or in the case of penicillin-resistant pneumococcal isolates (MIC ≥ 4.0 μg/mL) [49]. However, linezolid and levofloxacin could trigger severe adverse drug reactions (ADRs). Hence, they are absent in some guidelines for children, such as the British National Formulary (BNF) [51], European guidelines [52], and Canadian Paediatric Society [7].
Our study revealed that most S. pneumoniae strains isolated from 3 hospitals were still susceptible to β-lactam antibiotics. In Vietnam, the rate of β-lactam resistant S. pneumoniae has still been relatively lower than in some Asian countries, such as China and Korea [53, 54]. The resistance mechanism of S. pneumoniae to β-lactams is the modification of PBPs (Penicillin-binding proteins), decreasing the affinity of β-lactams with their targets rather than the production of beta-lactamase [55].
It is worthy of note that there is no difference between the resistance rate to amoxicillin and co-amoxiclav. Therefore, in CAP’s case caused by S. pneumoniae, amoxicillin should be prescribed instead of co-amoxiclav to lower the treatment cost.
Noticeably, the data showed that cefotaxime demonstrated a better antimicrobial effect than ceftriaxone in the cephalosporin group. The susceptibility rate of pneumococcal isolates to cefotaxime was 89.3%, whereas this percentage for ceftriaxone was 51.8%. The ceftriaxone MIC of 96.4% strains was ≤ 2 μg/mL. The increase of ceftriaxone non-susceptible S. pneumoniae isolates in the present could be explained by the emergence and combination of mutations on three resistance genes, PBP1a, 2b, and 2x, especially PBP 2b, which is responsible for encoding ceftriaxone low-affinity PBPs [56, 57].
Nowadays, multidrug-resistant S. pneumoniae is an issue of concern as well. Our research also showed that 47 of 56 pneumococcal strains were resistant to multiple antibiotics, representing 83.9%. In Vietnam, among 84 strains isolated from patients under five years old with lower respiratory tract infections, the rate of S. pneumoniae resistance to one or more antibiotics was 96%, 78% of which was multidrug-resistant pneumococci [58]. Based on a study in Thailand in 2014, the multidrug-resistance rate of S. pneumonia was 31.6% [59]. Pneumococcal MDR has many causes, primarily due to widespread use and abuse of antibiotics in the community, unnecessary prescribing by physicians and patients who do not adhere to the regime [60].
Until now, β-lactams have shown a powerful antimicrobial effect on S. pneumoniae isolates in Da Nang city and Quang Nam province. Hence, they should be the first choice for treating CAP in children. However, in cases of reduced susceptibility to β-lactams antibiotics, dose adjustment is required based on pharmacokinetics/pharmacodynamics, and antimicrobial therapy must be used reasonably to not increase the future resistance rate.
In addition to the appropriate use of antibiotics in clinical settings, the widespread use of pneumococcal conjugate vaccines such as PCV-7 or PCV-13 is significantly essential to lower the antimicrobial resistance of S. pneumoniae. The introduction of PCV7 and PCV-13 in the USA in the first decade of the 21st has remarkably reduced antibiotic-resistant pneumococcal infections [61]. Although the emergence of non-vaccine serotypes might reduce the effectiveness of pneumococcal vaccines [61, 62], they have still played a pivotal role in fighting pneumococcal diseases and tackling the problem of antibiotic resistance. Therefore, PCV-7 and PCV-13 should be introduced in the Expanded Vaccination Program in Vietnam.
Our study has some limitations. The only nasopharyngeal aspirate specimen was used to isolate the organisms. As the nature of a fastidious organism (S. pneumoniae) that was vulnerable to changing temperature, most of the missing strains in our study were due to cell death during the transportation in long distances.
Conclusion
Our study showed updated data of CAP children caused by S. pneumoniae in Da Nang and Quang Nam. It would be significantly beneficial for further studies, particularly the investigations on the effectiveness of PCV, to design and run an appropriate vaccination program.
Our data advocate the use of penicillin G, amoxicillin, co-amoxiclav, or cefotaxime as the first-line therapy for uncomplicated S. pneumonia-induced CAP in children Quang Nam – Da Nang. Vancomycin, linezolid, and levofloxacin should be used as alternatives or for the infections caused by multi-drug resistant S. pneumoniae. Due to a high prevalence of macrolide-resistant S. pneumoniae, a macrolide empirical monotherapy is not recommended for the management of CAP unless atypical microorganisms are suspected. Because the prevalence of multidrug-resistant pneumococci in Da Nang and Quang Nam (83.9%) is an issue of concern, it is required to make a dramatic change in the antibiotic-prescribing habits of physicians, other health care workers, and the public about the proper and wise use of antibiotics.